444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug market is experiencing steady growth driven by the increasing prevalence of HIV/AIDS, advancements in antiretroviral therapy (ART), and the introduction of fixed-dose combination therapies for simplified treatment regimens. This combination drug, often referred to as Tenofovir/Lamivudine/Atazanavir/Ritonavir or Tenofovir/Lamivudine/Atazanavir/R, is widely used in the management of HIV infection as part of highly active antiretroviral therapy (HAART). By combining multiple antiretroviral agents with complementary mechanisms of action, this drug regimen offers enhanced efficacy, reduced pill burden, and improved adherence, leading to better patient outcomes and disease management.
Meaning
The Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug is a fixed-dose combination therapy used in the treatment of HIV/AIDS. Tenofovir and Lamivudine are nucleoside reverse transcriptase inhibitors (NRTIs) that inhibit the replication of HIV by interfering with the enzyme reverse transcriptase. Atazanavir is a protease inhibitor that blocks the activity of the HIV protease enzyme, preventing the virus from producing mature infectious particles. Ritonavir is a pharmacokinetic enhancer that boosts the levels of Atazanavir in the bloodstream, allowing for lower doses and improved drug efficacy. Together, these four drugs work synergistically to suppress viral replication, reduce viral load, and improve immune function in HIV-infected individuals.
Executive Summary
The Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug market is characterized by steady demand driven by the ongoing global HIV/AIDS pandemic and the need for effective antiretroviral therapy. With millions of people living with HIV worldwide, there is a growing demand for safe, effective, and affordable treatment options that can suppress viral replication, prevent disease progression, and improve quality of life. The introduction of fixed-dose combination therapies such as Tenofovir/Lamivudine/Atazanavir/R has revolutionized HIV treatment by simplifying drug regimens, reducing pill burden, and enhancing treatment adherence, thereby contributing to better long-term outcomes for patients.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug market is characterized by dynamic trends and evolving treatment guidelines. Rapid advancements in drug development, clinical research, and public health policies influence market dynamics. Key factors driving market growth include the increasing adoption of combination therapy regimens as first-line treatment for HIV/AIDS, the expansion of treatment access in low- and middle-income countries, and the development of long-acting formulations and novel drug delivery technologies to improve treatment adherence and simplify dosing schedules.
Regional Analysis
The Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug market exhibits regional variations, with high-income countries in North America, Europe, and Asia-Pacific leading in terms of market size and treatment coverage. However, low- and middle-income countries in sub-Saharan Africa, South Asia, and Latin America bear the highest burden of HIV/AIDS and face significant challenges in accessing and affording antiretroviral therapy. Market expansion in these regions is driven by government-led treatment programs, international aid initiatives, and efforts to strengthen healthcare infrastructure and capacity for HIV care and management.
Competitive Landscape
Leading Companies in the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug market can be segmented based on drug formulation, dosage strength, patient population, and geography. Formulations include oral tablets, capsules, and oral solutions. Dosage strengths vary depending on the individual drugs’ potency and pharmacokinetic properties. Patient populations include treatment-naive individuals, treatment-experienced patients, pediatric patients, pregnant women, and individuals co-infected with other pathogens such as hepatitis B or hepatitis C virus.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug market, disrupting treatment access, delivery, and monitoring services for HIV/AIDS patients worldwide. While efforts to maintain essential health services and ensure uninterrupted antiretroviral therapy have been prioritized, the pandemic has strained healthcare systems, diverted resources, and exacerbated existing challenges in HIV care and management. However, the pandemic has also highlighted the importance of resilient health systems, community engagement, and innovative approaches to HIV service delivery, paving the way for future improvements in HIV/AIDS care and treatment.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug market is expected to continue growing, driven by factors such as increasing treatment access, expanding treatment guidelines, and ongoing investments in HIV/AIDS research and development. With the global commitment to ending the HIV/AIDS pandemic by 2030, there is a renewed focus on scaling up prevention, testing, treatment, and care services to reach all affected populations and achieve epidemic control. By leveraging advances in drug development, healthcare delivery, and public health strategies, stakeholders in the Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug market can contribute to the global effort to eliminate HIV/AIDS as a public health threat.
Conclusion
In conclusion, the Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug market plays a critical role in the global response to the HIV/AIDS pandemic, providing safe, effective, and affordable treatment options for millions of people living with HIV worldwide. As efforts to expand treatment access, improve treatment outcomes, and prevent new infections continue, the demand for innovative combination therapies such as Tenofovir/Lamivudine/Atazanavir/R is expected to grow. By addressing challenges such as treatment access, affordability, and treatment adherence, stakeholders in the Tenofovir, Lamivudine, Atazanavir, and Ritonavir combination drug market can contribute to achieving the goal of ending the HIV/AIDS pandemic and ensuring health and well-being for all.
What is Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug?
Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug refers to a therapeutic regimen used primarily in the treatment of HIV. This combination aims to enhance antiviral efficacy while minimizing resistance and side effects.
What are the key players in the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market?
Key players in the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market include Gilead Sciences, AbbVie, and Mylan, among others.
What are the growth factors driving the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market?
The growth of the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market is driven by the increasing prevalence of HIV infections, advancements in drug formulations, and rising awareness about HIV treatment options.
What challenges does the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market face?
Challenges in the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market include the potential for drug resistance, high costs of treatment, and regulatory hurdles in different regions.
What opportunities exist in the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market?
Opportunities in the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market include the development of new formulations, expansion into emerging markets, and increasing collaborations between pharmaceutical companies and healthcare providers.
What trends are shaping the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market?
Trends in the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market include the shift towards personalized medicine, the integration of digital health technologies, and a focus on long-acting injectable formulations.
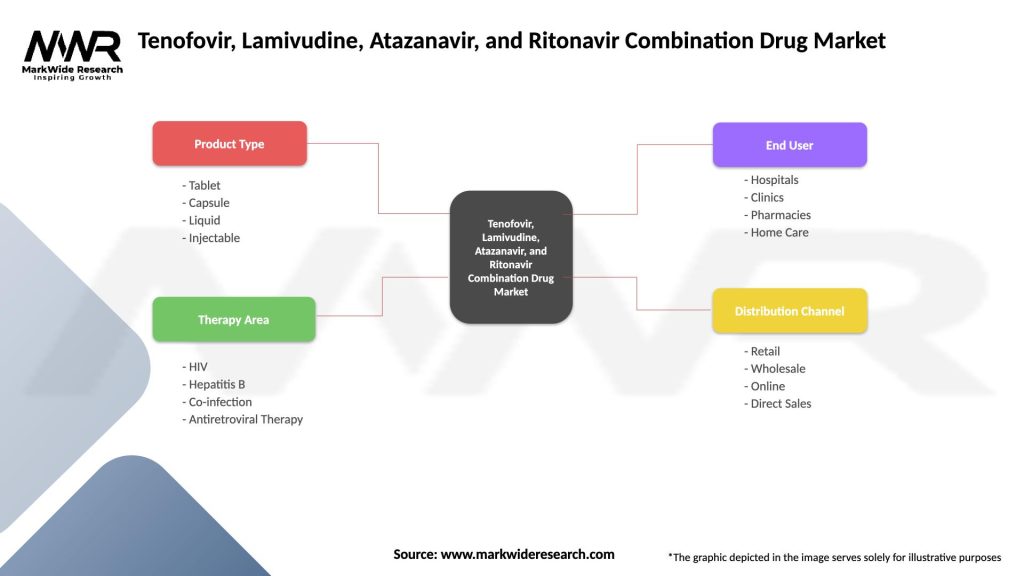
Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market
| Segmentation Details | Description |
|---|---|
| Product Type | Tablet, Capsule, Liquid, Injectable |
| Therapy Area | HIV, Hepatitis B, Co-infection, Antiretroviral Therapy |
| End User | Hospitals, Clinics, Pharmacies, Home Care |
| Distribution Channel | Retail, Wholesale, Online, Direct Sales |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Tenofovir, Lamivudine, Atazanavir, and Ritonavir Combination Drug Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at