444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview: The Clomiphene Citrate Market is a significant segment within the pharmaceutical industry, catering to individuals facing fertility issues. Clomiphene citrate is a commonly prescribed medication used to stimulate ovulation in women who have trouble conceiving due to ovulatory dysfunction. It works by inducing the release of hormones necessary for ovulation, thus increasing the chances of pregnancy. The market for clomiphene citrate is driven by the rising prevalence of infertility, increasing awareness about fertility treatments, and advancements in reproductive medicine.
Meaning: Clomiphene citrate, often marketed under the brand name Clomid, is a selective estrogen receptor modulator (SERM) that is widely used in the treatment of female infertility. It is taken orally and acts by blocking estrogen receptors in the hypothalamus, leading to an increase in the production of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). This, in turn, stimulates the ovaries to produce eggs, thereby improving the chances of conception in women struggling with ovulatory disorders.
Executive Summary: The Clomiphene Citrate Market is witnessing steady growth, fueled by factors such as increasing infertility rates, advancements in assisted reproductive technologies, and the growing acceptance of fertility treatments. Pharmaceutical companies are investing in research and development to enhance the efficacy and safety profile of clomiphene citrate formulations, thereby expanding its application in the treatment of infertility. Moreover, collaborations between healthcare providers and fertility clinics are facilitating greater access to clomiphene citrate therapy for patients worldwide.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics: The Clomiphene Citrate Market operates within a dynamic landscape shaped by changing demographics, evolving healthcare policies, and advancements in reproductive science. Market players must navigate regulatory complexities, address safety concerns, and explore innovative strategies to capitalize on emerging trends and opportunities in the global fertility market.
Regional Analysis: The demand for clomiphene citrate varies across regions due to differences in healthcare infrastructure, cultural attitudes toward fertility, and regulatory frameworks governing reproductive medicine. Developed regions such as North America and Europe account for a significant share of the market, driven by higher healthcare spending and greater awareness about fertility treatments. However, emerging markets in Asia Pacific, Latin America, and Middle East offer lucrative growth prospects, fueled by rising infertility rates and improving access to healthcare services.
Competitive Landscape
Leading Companies in the Clomiphene Citrate Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
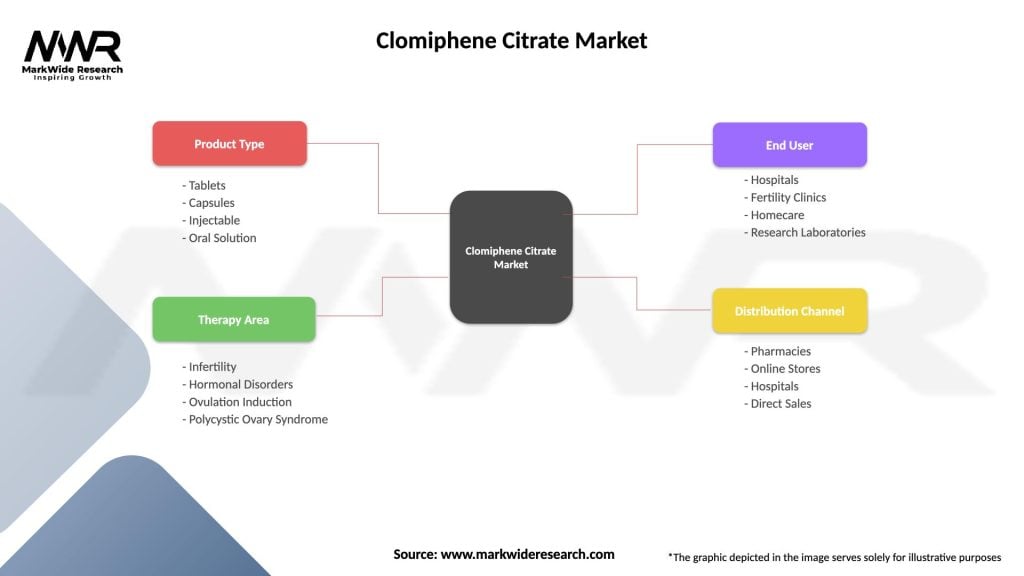
Segmentation: The Clomiphene Citrate Market can be segmented based on factors such as formulation type, dosage strength, distribution channel, and geographical regions. Common segmentation categories include oral tablets, extended-release formulations, fertility clinics, hospitals, retail pharmacies, North America, Europe, Asia Pacific, among others.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
Market Key Trends:
Covid-19 Impact: The Covid-19 pandemic has had a significant impact on the Clomiphene Citrate Market, disrupting fertility services, delaying elective treatments, and affecting patient access to reproductive healthcare. However, as healthcare systems adapt to the new normal and fertility clinics resume operations, the demand for clomiphene citrate and other fertility drugs is expected to rebound, driven by pent-up demand, postponed treatment cycles, and increasing awareness about fertility preservation options.
Key Industry Developments:
Analyst Suggestions:
Future Outlook: The future outlook for the Clomiphene Citrate Market is promising, driven by factors such as increasing infertility rates, technological advancements in reproductive medicine, and expanding access to fertility treatments worldwide. Despite challenges such as regulatory constraints, safety concerns, and market competition, strategic initiatives focused on innovation, patient-centric care, and regulatory compliance will drive market growth, differentiation, and value creation for industry stakeholders.
Conclusion: In conclusion, the Clomiphene Citrate Market plays a crucial role in addressing the unmet needs of individuals struggling with infertility, offering a safe, effective, and accessible option for ovulation induction therapy. With rising infertility rates, increasing awareness about fertility treatments, and advancements in reproductive medicine, the demand for clomiphene citrate is expected to grow steadily in the coming years. However, market players must navigate regulatory complexities, address safety concerns, and innovate to capitalize on emerging trends and opportunities in the global fertility market, ensuring continued growth, relevance, and sustainability in the Clomiphene Citrate Market ecosystem.
What is Clomiphene Citrate?
Clomiphene Citrate is a medication primarily used to treat infertility in women by inducing ovulation. It works by blocking estrogen receptors in the hypothalamus, which increases the release of hormones that stimulate the ovaries.
What are the key companies in the Clomiphene Citrate Market?
Key companies in the Clomiphene Citrate Market include Merck & Co., Inc., Teva Pharmaceutical Industries Ltd., and Mylan N.V., among others.
What are the growth factors driving the Clomiphene Citrate Market?
The Clomiphene Citrate Market is driven by increasing infertility rates, rising awareness about fertility treatments, and advancements in reproductive health technologies. Additionally, the growing acceptance of assisted reproductive technologies contributes to market growth.
What challenges does the Clomiphene Citrate Market face?
The Clomiphene Citrate Market faces challenges such as potential side effects associated with the medication, competition from alternative fertility treatments, and regulatory hurdles. These factors can impact the adoption and market penetration of Clomiphene Citrate.
What opportunities exist in the Clomiphene Citrate Market?
Opportunities in the Clomiphene Citrate Market include the development of new formulations and combination therapies, expanding into emerging markets, and increasing collaborations between pharmaceutical companies and fertility clinics. These factors can enhance market reach and patient access.
What trends are shaping the Clomiphene Citrate Market?
Trends in the Clomiphene Citrate Market include a growing preference for oral medications over injectable treatments, increased focus on personalized medicine, and the integration of telemedicine in fertility consultations. These trends are influencing how treatments are delivered and accessed.
Clomiphene Citrate Market
| Segmentation Details | Description |
|---|---|
| Product Type | Tablets, Capsules, Injectable, Oral Solution |
| Therapy Area | Infertility, Hormonal Disorders, Ovulation Induction, Polycystic Ovary Syndrome |
| End User | Hospitals, Fertility Clinics, Homecare, Research Laboratories |
| Distribution Channel | Pharmacies, Online Stores, Hospitals, Direct Sales |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Clomiphene Citrate Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at