444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview: The Lightproof Infusion Set Market encompasses a range of medical devices designed for the administration of intravenous fluids, medications, and blood products while protecting light-sensitive medications from photodegradation. These infusion sets are essential tools used in healthcare settings such as hospitals, clinics, and home care settings to deliver fluids and medications safely and accurately to patients. The market for lightproof infusion sets is driven by the increasing prevalence of chronic diseases requiring long-term infusion therapy, advancements in medical technology, and the growing demand for patient-centric healthcare solutions.
Meaning: Lightproof infusion sets are medical devices used to administer intravenous fluids, medications, and blood products to patients while protecting light-sensitive medications from degradation due to exposure to ambient light. These infusion sets typically consist of components such as tubing, a drip chamber, a flow regulator, and a needle or catheter for insertion into the patient’s vein. Lightproof infusion sets are essential for delivering medications such as antibiotics, chemotherapy drugs, and blood products that are susceptible to degradation when exposed to light, ensuring the safety and efficacy of patient care.
Executive Summary: The Lightproof Infusion Set Market is driven by factors such as the increasing prevalence of chronic diseases requiring long-term infusion therapy, advancements in medical technology, and the growing emphasis on patient safety and quality of care. Market players focus on innovation to develop advanced infusion set designs with enhanced light protection features, improved flow control mechanisms, and better patient comfort. Collaborations between manufacturers, healthcare providers, and regulatory agencies drive the development of standardized infusion set guidelines and protocols, ensuring safe and effective infusion therapy for patients worldwide.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics: The Lightproof Infusion Set Market operates in a dynamic environment shaped by factors such as disease prevalence, technological advancements, regulatory compliance, and healthcare infrastructure. Market players must navigate these dynamics effectively to capitalize on growth opportunities, address market challenges, and deliver innovative solutions that meet the needs of patients and healthcare providers worldwide.
Regional Analysis: The demand for lightproof infusion sets varies by region, influenced by factors such as healthcare infrastructure, disease prevalence, regulatory frameworks, and reimbursement policies. Developed regions with advanced healthcare systems and higher disease burdens, such as North America and Europe, dominate the market, while emerging markets in Asia Pacific and Latin America present growth opportunities due to increasing healthcare spending and rising awareness of infusion therapy benefits.
Competitive Landscape:
Leading Companies: Lightproof Infusion Set Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
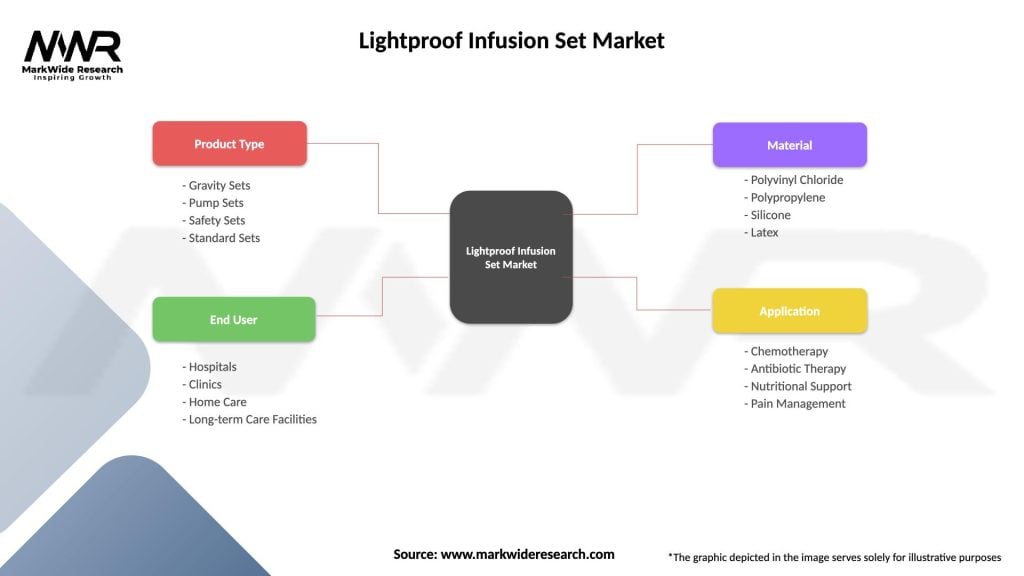
Segmentation: The Lightproof Infusion Set Market can be segmented based on factors such as infusion set type, application, end-user, and geography. Common infusion set types include gravity infusion sets, syringe infusion sets, and pump infusion sets, each designed for specific infusion therapy requirements and patient needs. Applications range from intravenous fluid administration to medication infusion and blood product transfusion in various healthcare settings.
Category-wise Insight: Lightproof infusion sets are essential tools used in intravenous therapy to deliver fluids, medications, and blood products safely and accurately to patients while protecting light-sensitive medications from degradation. These infusion sets complement other medical devices and techniques in patient care, contributing to improved treatment outcomes and patient safety in healthcare settings worldwide.
Key Benefits for Industry Participants and Stakeholders: The Lightproof Infusion Set Market offers several benefits for industry participants and stakeholders, including:
SWOT Analysis: A SWOT analysis provides insights into the strengths, weaknesses, opportunities, and threats facing the Lightproof Infusion Set Market:
Market Key Trends:
Covid-19 Impact: The Covid-19 pandemic has reshaped healthcare delivery and patient care practices, including infusion therapy protocols and lightproof infusion set utilization. Healthcare facilities have implemented infection control measures, telemedicine services, and procedural protocols to ensure patient safety and continuity of care while managing pandemic-related challenges such as supply chain disruptions, workforce shortages, and increased patient volumes.
Key Industry Developments:
Analyst Suggestions:
Future Outlook: The Lightproof Infusion Set Market is poised for steady growth and innovation driven by increasing disease prevalence, technological advancements, and patient safety emphasis in healthcare delivery. Market players have opportunities to capitalize on emerging trends, address unmet needs, and drive market expansion through innovation, education, and regulatory compliance to deliver value-added solutions that meet the evolving needs of patients and healthcare providers worldwide.
Conclusion: Lightproof infusion sets play a crucial role in intravenous therapy, enabling healthcare providers to deliver fluids, medications, and blood products safely and accurately to patients while protecting light-sensitive medications from degradation. The Lightproof Infusion Set Market offers opportunities for manufacturers, healthcare providers, and stakeholders to enhance patient safety, improve treatment outcomes, and contribute to the advancement of infusion therapy worldwide. By focusing on innovation, education, and regulatory compliance, industry players can drive market growth, address unmet needs, and deliver value-added solutions that meet the evolving needs of patients and healthcare providers in a dynamic healthcare landscape.
What is Lightproof Infusion Set?
Lightproof infusion sets are medical devices designed to deliver fluids, medications, or nutrients to patients while protecting the contents from light exposure. This is crucial for certain medications that can degrade when exposed to light, ensuring patient safety and treatment efficacy.
What are the key players in the Lightproof Infusion Set Market?
Key players in the Lightproof Infusion Set Market include B. Braun Melsungen AG, Baxter International Inc., and Terumo Corporation, among others. These companies are known for their innovative products and extensive distribution networks in the healthcare sector.
What are the growth factors driving the Lightproof Infusion Set Market?
The growth of the Lightproof Infusion Set Market is driven by the increasing prevalence of chronic diseases requiring long-term infusion therapy, advancements in medical technology, and a growing emphasis on patient safety in healthcare settings.
What challenges does the Lightproof Infusion Set Market face?
Challenges in the Lightproof Infusion Set Market include stringent regulatory requirements, the high cost of advanced infusion systems, and competition from alternative drug delivery methods. These factors can hinder market growth and innovation.
What opportunities exist in the Lightproof Infusion Set Market?
Opportunities in the Lightproof Infusion Set Market include the development of smart infusion systems with integrated monitoring capabilities, increasing demand for home healthcare solutions, and expanding applications in oncology and critical care.
What trends are shaping the Lightproof Infusion Set Market?
Trends in the Lightproof Infusion Set Market include the rise of personalized medicine, the integration of digital health technologies, and a focus on sustainability in product design. These trends are influencing how infusion sets are developed and utilized in clinical practice.
Lightproof Infusion Set Market
| Segmentation Details | Description |
|---|---|
| Product Type | Gravity Sets, Pump Sets, Safety Sets, Standard Sets |
| End User | Hospitals, Clinics, Home Care, Long-term Care Facilities |
| Material | Polyvinyl Chloride, Polypropylene, Silicone, Latex |
| Application | Chemotherapy, Antibiotic Therapy, Nutritional Support, Pain Management |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies: Lightproof Infusion Set Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at