444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The retinal inflammation treatment drug market encompasses pharmaceuticals developed for managing and alleviating inflammatory conditions affecting the retina. Retinal inflammation, also known as uveitis, poses significant challenges to vision and ocular health, necessitating effective treatment approaches. This market caters to the diverse needs of patients suffering from various forms of retinal inflammation, offering therapeutic options to mitigate symptoms, prevent complications, and preserve visual function.
Meaning
Retinal inflammation, or uveitis, refers to the inflammation of the uvea, which includes the iris, ciliary body, and choroid, in addition to the adjacent retina. It can arise from various etiologies, including autoimmune disorders, infectious agents, trauma, and systemic diseases. Effective treatment of retinal inflammation aims to suppress ocular inflammation, alleviate symptoms such as pain and redness, prevent complications like macular edema and retinal detachment, and preserve visual acuity and quality of life for affected individuals.
Executive Summary
The retinal inflammation treatment drug market is witnessing steady growth, driven by the increasing prevalence of uveitis, advancements in treatment modalities, and the expanding pipeline of novel therapeutics targeting inflammatory pathways. Key market players are investing in research and development, clinical trials, and strategic collaborations to address unmet medical needs, improve treatment outcomes, and enhance patient care in the field of retinal inflammation. However, challenges such as regulatory hurdles, limited treatment options for refractory cases, and the high cost of biologic therapies may pose barriers to market growth and adoption.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The retinal inflammation treatment drug market operates in a dynamic environment influenced by various factors, including disease epidemiology, treatment innovations, regulatory landscapes, and patient preferences. These dynamics shape market trends, opportunities, and challenges, requiring stakeholders to adapt and innovate to meet evolving market demands and unmet medical needs.
Regional Analysis
The retinal inflammation treatment drug market exhibits regional variations in disease burden, treatment practices, regulatory frameworks, and market dynamics. Key regions driving market growth include:
Competitive Landscape
Leading Companies in Retinal Inflammation Treatment Drug Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
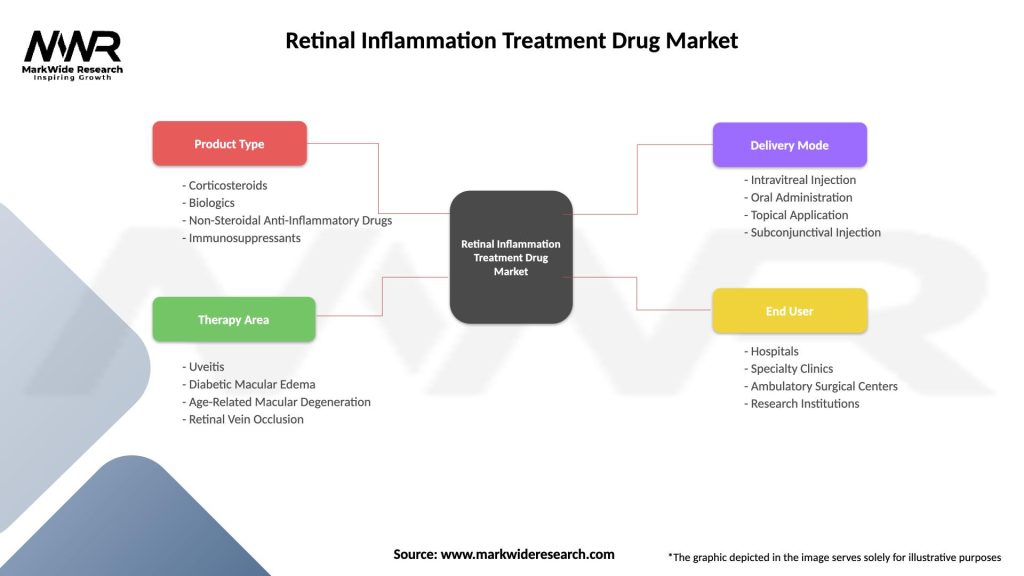
Segmentation
The retinal inflammation treatment drug market can be segmented based on various factors, including:
Segmentation provides a comprehensive understanding of market dynamics, patient needs, and therapeutic options available for managing retinal inflammation, guiding treatment decisions, and optimizing patient outcomes.
Category-wise Insight
Each category of retinal inflammation treatment drugs offers unique benefits, mechanisms of action, and considerations for clinical use, guiding treatment decisions and optimizing therapeutic outcomes for patients with uveitis.
Key Benefits for Industry Participants and Stakeholders
These benefits translate into improved clinical outcomes, patient satisfaction, and healthcare resource utilization, driving market adoption and investment in retinal inflammation treatment drugs.
SWOT Analysis
Strengths
Weaknesses
Opportunities
Threats
Understanding these factors through a SWOT analysis helps stakeholders identify strategic opportunities, address weaknesses, mitigate threats, and capitalize on market strengths to drive innovation, growth, and competitiveness in the dynamic retinal inflammation treatment drug market.
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the retinal inflammation treatment drug market, influencing disease management, treatment practices, and market dynamics. Key impacts of COVID-19 on the market include:
Despite these challenges, the retinal inflammation treatment drug market demonstrated resilience and adaptability, with stakeholders leveraging digital health solutions, collaborative research initiatives, and innovative treatment approaches to navigate the impact of COVID-19 and continue advancing patient care in the field of retinal inflammation.
Key Industry Developments
These industry developments underscore the commitment of key stakeholders to advancing patient care, driving innovation, and addressing unmet medical needs in the field of retinal inflammation treatment.
Analyst Suggestions
Future Outlook
The retinal inflammation treatment drug market is poised for continued growth and innovation, driven by factors such as increasing disease prevalence, advancements in treatment modalities, expanding research and development efforts, and collaborative industry initiatives. Key trends shaping the future outlook of the market include the development of novel therapeutics targeting specific inflammatory pathways, advancements in drug delivery technologies, adoption of personalized medicine approaches, and expansion into emerging markets.
Stakeholders in the retinal inflammation treatment drug market should remain vigilant to evolving market dynamics, regulatory landscapes, and patient needs, while continuing to invest in research, innovation, and strategic collaborations to address unmet medical needs, improve treatment outcomes, and advance patient care in the field of retinal inflammation.
Conclusion
In conclusion, the retinal inflammation treatment drug market is positioned for significant growth and innovation in the coming years. With increasing disease prevalence, advancements in treatment modalities, and expanding research and development efforts, stakeholders have ample opportunities to address unmet medical needs and improve patient outcomes.
Despite challenges such as regulatory hurdles, competition from biosimilars, and disruptions caused by the COVID-19 pandemic, the market has demonstrated resilience and adaptability. Stakeholders have leveraged digital health solutions, collaborative research initiatives, and innovative treatment approaches to navigate these challenges and continue advancing patient care.
Looking ahead, investment in research and development, robust clinical trial design, regulatory engagement, and patient-centric approaches will be key to sustaining market growth and addressing evolving market dynamics. By remaining vigilant to emerging trends, regulatory landscapes, and patient needs, stakeholders can drive innovation, improve treatment efficacy, and enhance quality of life for patients with retinal inflammation.
What is Retinal Inflammation Treatment Drug?
Retinal Inflammation Treatment Drug refers to medications specifically designed to reduce inflammation in the retina, which can be caused by various conditions such as uveitis or diabetic retinopathy. These drugs aim to preserve vision and improve the quality of life for affected patients.
What are the key players in the Retinal Inflammation Treatment Drug Market?
Key players in the Retinal Inflammation Treatment Drug Market include companies like Novartis, Regeneron Pharmaceuticals, and Roche, which are known for their innovative therapies and research in ophthalmology, among others.
What are the main drivers of growth in the Retinal Inflammation Treatment Drug Market?
The growth of the Retinal Inflammation Treatment Drug Market is driven by the increasing prevalence of retinal diseases, advancements in drug formulations, and a growing aging population that is more susceptible to eye conditions.
What challenges does the Retinal Inflammation Treatment Drug Market face?
Challenges in the Retinal Inflammation Treatment Drug Market include high development costs, stringent regulatory requirements, and competition from alternative therapies that may limit market growth.
What opportunities exist in the Retinal Inflammation Treatment Drug Market?
Opportunities in the Retinal Inflammation Treatment Drug Market include the development of novel therapies targeting specific inflammatory pathways and the potential for combination treatments that enhance efficacy and patient outcomes.
What trends are shaping the Retinal Inflammation Treatment Drug Market?
Trends in the Retinal Inflammation Treatment Drug Market include a focus on personalized medicine, increased investment in research and development, and the integration of digital health technologies to monitor treatment responses.
Retinal Inflammation Treatment Drug Market
| Segmentation Details | Description |
|---|---|
| Product Type | Corticosteroids, Biologics, Non-Steroidal Anti-Inflammatory Drugs, Immunosuppressants |
| Therapy Area | Uveitis, Diabetic Macular Edema, Age-Related Macular Degeneration, Retinal Vein Occlusion |
| Delivery Mode | Intravitreal Injection, Oral Administration, Topical Application, Subconjunctival Injection |
| End User | Hospitals, Specialty Clinics, Ambulatory Surgical Centers, Research Institutions |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Retinal Inflammation Treatment Drug Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at