444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Oridonin Market encompasses the pharmaceutical industry’s interest in the therapeutic potential of oridonin, a diterpenoid compound derived from Rabdosia rubescens, a traditional Chinese medicinal herb. Oridonin has garnered attention for its diverse pharmacological properties, including anticancer, anti-inflammatory, antioxidant, and immunomodulatory effects, making it a promising candidate for drug development and clinical research.
Meaning
Oridonin, also known as Rubescenin or Isodonol, is a natural compound isolated from Rabdosia rubescens, a perennial plant native to China, Japan, and Korea. Used in traditional Chinese medicine for centuries, oridonin exhibits potent biological activities and therapeutic potential against various diseases, particularly cancer. Its unique chemical structure and multifaceted pharmacological effects have sparked scientific interest in exploring its medicinal properties and therapeutic applications.
Executive Summary
The Oridonin Market is driven by growing research interest in natural products-based drug discovery, rising prevalence of cancer and inflammatory diseases, and increasing investment in pharmaceutical research and development. Oridonin’s versatile pharmacological profile, favorable safety profile, and potential synergies with conventional cancer therapies position it as a promising candidate for anticancer drug development and personalized medicine approaches. However, challenges such as limited bioavailability, formulation issues, and regulatory hurdles pose barriers to its clinical translation and commercialization.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Oridonin Market operates within a dynamic landscape shaped by scientific advancements, regulatory requirements, market trends, and competitive dynamics. Key market dynamics include:
Regional Analysis
The Oridonin Market exhibits regional variations in research activity, clinical trials, regulatory frameworks, and market dynamics. Key regions driving market growth and innovation include:
Competitive Landscape
Leading Companies in Oridonin Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
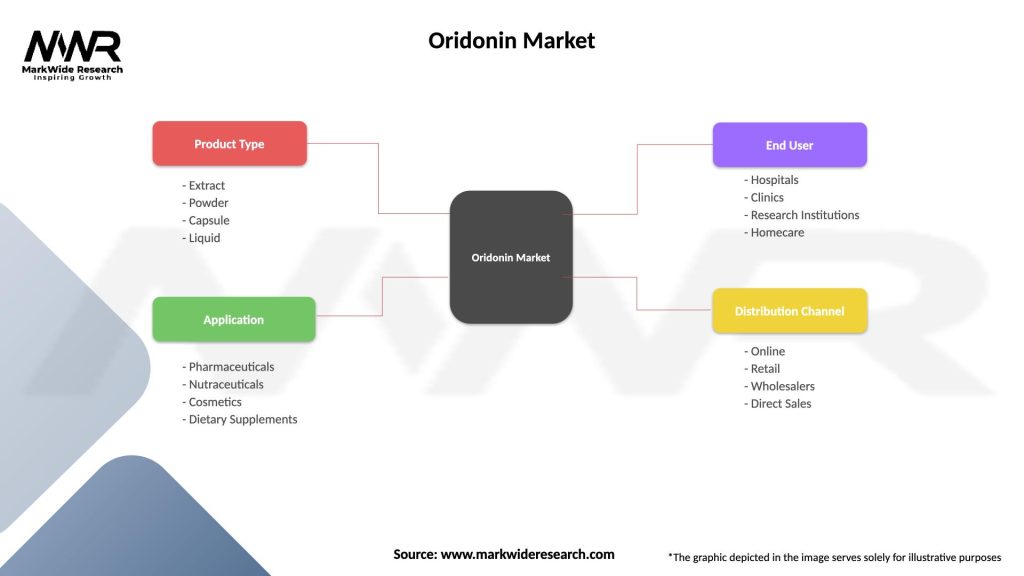
Segmentation
The Oridonin Market can be segmented based on:
Segmentation provides insights into the diverse applications, formulations, and stages of development of oridonin-based drugs, enabling stakeholders to tailor their strategies and investments to specific market segments and therapeutic opportunities.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
A SWOT analysis provides insights into the strengths, weaknesses, opportunities, and threats facing the Oridonin Market:
Understanding these internal and external factors through a SWOT analysis enables stakeholders to capitalize on strengths, address weaknesses, leverage opportunities, and mitigate threats in the dynamic Oridonin Market landscape.
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has impacted the Oridonin Market in several ways:
Key Industry Developments
Analyst Suggestions
Analysts offer the following suggestions for stakeholders in the Oridonin Market:
Future Outlook
The future outlook for the Oridonin Market is promising, with several key trends and developments shaping the trajectory of this dynamic industry:
Overall, the future outlook for the Oridonin Market is characterized by optimism, innovation, and collaboration, as stakeholders across the pharmaceutical industry, healthcare sector, and regulatory agencies work together to unlock the full therapeutic potential of oridonin and improve patient care in oncology, inflammation, neurology, and other disease areas. By leveraging technological advancements, embracing precision medicine approaches, and fostering collaborative research partnerships, the Oridonin Market will continue to evolve and flourish, delivering innovative solutions and transformative therapies to patients worldwide.
Conclusion
The Oridonin Market presents significant opportunities and challenges for stakeholders in the pharmaceutical industry, driven by growing research interest in natural products-based drug discovery, rising prevalence of cancer and inflammatory diseases, and increasing investment in precision medicine and personalized therapy approaches. Oridonin’s versatile pharmacological profile, therapeutic potential in oncology, inflammation, and neurology, and collaborative research efforts position it as a promising candidate for drug development and clinical translation, pending further preclinical and clinical validation. Addressing formulation challenges, regulatory hurdles, and market dynamics requires interdisciplinary collaboration, innovative drug delivery strategies, and precision medicine approaches to harness oridonin’s therapeutic benefits and improve patient outcomes in diverse disease indications. As research advances, clinical trials progress, and regulatory approvals are obtained, the Oridonin Market holds promise for delivering novel therapeutic solutions and advancing precision medicine paradigms in the global healthcare landscape.
What is Oridonin?
Oridonin is a natural compound derived from the plant Rabdosia rubescens, known for its potential therapeutic properties, including anti-inflammatory and anti-cancer effects. It is primarily studied for its applications in traditional medicine and modern pharmacology.
What are the key players in the Oridonin Market?
Key players in the Oridonin Market include companies such as Chengdu Huirun Biotechnology Co., Ltd., and Xi’an Lyphar Biotech Co., Ltd., which are involved in the extraction and commercialization of Oridonin for various applications, among others.
What are the growth factors driving the Oridonin Market?
The Oridonin Market is driven by increasing research into natural compounds for therapeutic uses, rising demand for herbal medicines, and growing awareness of the health benefits associated with Oridonin. Additionally, its potential applications in cancer treatment and anti-inflammatory therapies contribute to market growth.
What challenges does the Oridonin Market face?
The Oridonin Market faces challenges such as regulatory hurdles regarding the approval of natural compounds for medical use and the need for extensive clinical trials to validate efficacy and safety. Additionally, competition from synthetic alternatives can impact market dynamics.
What opportunities exist in the Oridonin Market?
Opportunities in the Oridonin Market include expanding research into its medicinal properties, potential collaborations with pharmaceutical companies, and increasing consumer interest in natural and organic products. The growing trend towards integrative medicine also presents avenues for market expansion.
What trends are shaping the Oridonin Market?
Trends shaping the Oridonin Market include a rising focus on natural ingredients in health products, increased investment in herbal research, and the development of innovative formulations that incorporate Oridonin. Additionally, the shift towards preventive healthcare is driving interest in natural compounds.
Oridonin Market
| Segmentation Details | Description |
|---|---|
| Product Type | Extract, Powder, Capsule, Liquid |
| Application | Pharmaceuticals, Nutraceuticals, Cosmetics, Dietary Supplements |
| End User | Hospitals, Clinics, Research Institutions, Homecare |
| Distribution Channel | Online, Retail, Wholesalers, Direct Sales |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Oridonin Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at