444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview
The United States Hospital Acquired Disease Testing Market is a critical facet of healthcare, focusing on testing solutions to identify and prevent hospital-acquired infections (HAIs). This market plays a pivotal role in maintaining patient safety, improving infection control, and ensuring the quality of healthcare services. In this comprehensive analysis, we delve into the United States Hospital Acquired Disease Testing Market, exploring its meaning, executive summary, key market insights, market drivers, market restraints, market opportunities, market dynamics, regional analysis, competitive landscape, segmentation, category-wise insights, benefits for industry participants, SWOT analysis, market key trends, COVID-19 impact, key industry developments, analyst suggestions, future outlook, and a conclusive summary.
Meaning
The United States Hospital Acquired Disease Testing Market is dedicated to developing, manufacturing, and providing testing solutions for diseases acquired within healthcare settings. This market addresses the growing concern of hospital-acquired infections, focusing on rapid and accurate testing to control and prevent the spread of infections within hospitals.
Executive Summary
The United States Hospital Acquired Disease Testing Market is instrumental in safeguarding patients and healthcare professionals from nosocomial infections. Key market insights underscore the market’s role in ensuring patient safety, improving infection control measures, and advancing testing technologies. Technological advancements, stringent regulatory guidelines, and the emphasis on infection prevention drive market growth, positioning it as a critical component of the healthcare system.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Exploring the Crucial Elements of the United States Hospital Acquired Disease Testing Market
Market Drivers
Factors Fueling the Growth of the United States Hospital Acquired Disease Testing Market
Market Restraints
Market Opportunities
Market Dynamics
The United States Hospital Acquired Disease Testing Market operates within a dynamic healthcare landscape influenced by healthcare policies, emerging diseases, technological advancements, and the continuous need for infection prevention and control. As the healthcare sector evolves, the market adapts to provide innovative testing solutions and ensure patient safety.
Regional Analysis
The impact and relevance of hospital-acquired disease testing solutions vary across different states and regions within the United States, influenced by factors such as healthcare infrastructure, prevalence of infections, and healthcare policies. Regional analysis provides insights into market variations and growth potential.
Competitive Landscape
Leading Companies in the United States Hospital Acquired Disease Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
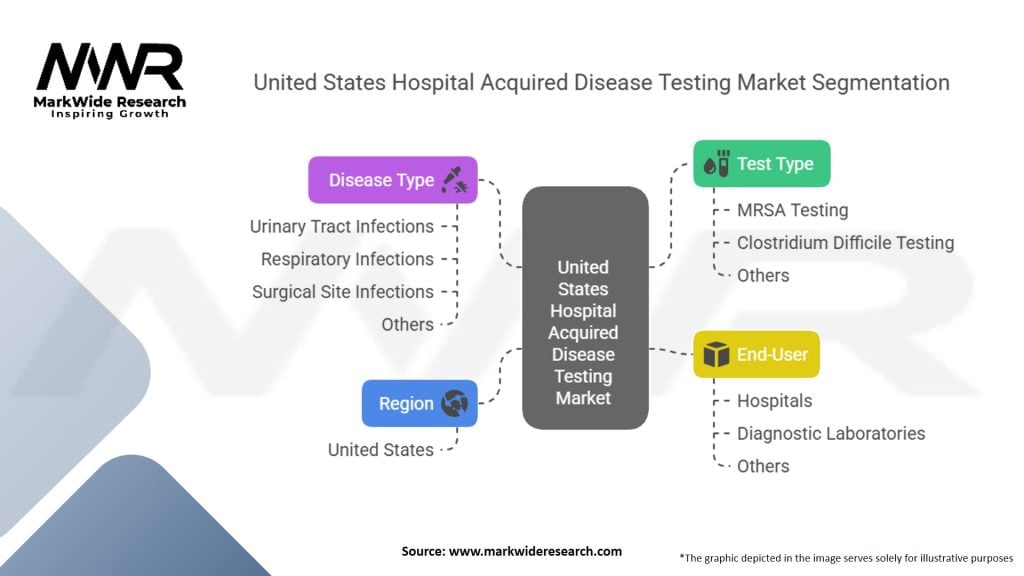
Segmentation
The United States Hospital Acquired Disease Testing Market in Detail
Category-wise Insights
Disease Type:
Respiratory Infections: Testing solutions for respiratory infections play a crucial role in identifying and preventing the spread of airborne diseases within healthcare settings.
Urinary Tract Infections (UTIs): UTI testing solutions are vital for early detection and management of urinary tract infections, a common nosocomial infection.
Bloodstream Infections: Testing for bloodstream infections is essential to prevent sepsis and other severe complications arising from hospital-acquired infections.
Technology:
Polymerase Chain Reaction (PCR): PCR-based testing solutions provide high sensitivity and specificity, making them effective for accurate disease diagnosis.
Immunoassays: Immunoassay-based testing methods are quick and efficient for the detection of disease-specific antibodies or antigens.
Molecular Diagnostics: Molecular diagnostics encompass a range of technologies that offer comprehensive disease testing and analysis at a molecular level.
Benefits for Industry Participants and Stakeholders
The Impact and Significance of the United States Hospital Acquired Disease Testing Market
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Identifying Trends Shaping the United States Hospital Acquired Disease Testing Market
COVID-19 Impact
The COVID-19 pandemic emphasized the importance of robust disease testing capabilities in healthcare. The pandemic led to an acceleration of testing technology development and a heightened focus on infection control, reinforcing the significance of the United States Hospital Acquired Disease Testing Market.
Key Industry Developments
Notable Developments Shaping the United States Hospital Acquired Disease Testing Market
Analyst Suggestions
Future Outlook
The United States Hospital Acquired Disease Testing Market is expected to witness sustained growth, driven by technological advancements, the rising prevalence of hospital-acquired infections, and the growing emphasis on patient safety. Industry participants will continue to play a vital role in enhancing disease testing solutions, ensuring healthcare quality, and contributing to infection prevention and control.
Conclusion
In conclusion, the United States Hospital Acquired Disease Testing Market is a critical component of the healthcare system, focusing on accurate and timely disease testing to enhance patient safety and infection control. Industry participants and stakeholders play a pivotal role in advancing testing technologies, ensuring efficient disease detection, and contributing to the overall quality of healthcare services. As the market evolves, it remains a fundamental pillar in the healthcare landscape, safeguarding patients and healthcare professionals from the risks of hospital-acquired infections.
United States Hospital Acquired Disease Testing Market:
| Segmentation Details | Description |
|---|---|
| Test Type | Methicillin-Resistant Staphylococcus Aureus (MRSA) Testing, Clostridium Difficile Testing, Others |
| Disease Type | Urinary Tract Infections, Respiratory Infections, Surgical Site Infections, Others |
| End-User | Hospitals, Diagnostic Laboratories, Others |
| Region | United States |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the United States Hospital Acquired Disease Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at