444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview
The US oral solid dosage contract manufacturing market is a critical component of the pharmaceutical industry, providing outsourcing services for the production of tablets, capsules, and other solid dosage forms. Contract manufacturing organizations (CMOs) offer expertise, capacity, and regulatory compliance to pharmaceutical companies seeking to outsource manufacturing operations to focus on research, development, and commercialization. The US market for oral solid dosage contract manufacturing is characterized by a diverse range of CMOs, serving both domestic and international clients across various therapeutic categories and dosage forms.
Meaning
Oral solid dosage contract manufacturing involves the outsourcing of tablet and capsule manufacturing services to specialized CMOs. Pharmaceutical companies leverage the capabilities and infrastructure of contract manufacturers to produce oral solid dosage forms efficiently and cost-effectively. Contract manufacturing allows pharmaceutical companies to access specialized expertise, state-of-the-art facilities, and regulatory compliance, enabling them to bring products to market faster and more efficiently than traditional in-house manufacturing.
Executive Summary
The US oral solid dosage contract manufacturing market is witnessing steady growth, driven by factors such as increasing demand for generic drugs, cost pressures, regulatory requirements, and outsourcing trends in the pharmaceutical industry. Contract manufacturing offers pharmaceutical companies flexibility, scalability, and cost savings by leveraging the capabilities of specialized CMOs. While the market faces challenges such as pricing pressures, quality concerns, and regulatory compliance, opportunities for growth exist in niche markets, complex formulations, and emerging therapeutic areas. To succeed in the competitive landscape, CMOs must focus on innovation, quality, and customer service to meet the evolving needs of pharmaceutical clients and maintain a competitive edge in the market.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The US oral solid dosage contract manufacturing market operates in a dynamic and competitive environment influenced by factors such as market trends, regulatory changes, technological advancements, and customer preferences. These dynamics shape market growth, innovation, and competition, requiring contract manufacturers to adapt, innovate, and differentiate to succeed in the rapidly evolving pharmaceutical landscape.
Regional Analysis
The US oral solid dosage contract manufacturing market exhibits regional variations in demand, concentration of contract manufacturing facilities, and customer preferences across different states and metropolitan areas. Regions with a high concentration of pharmaceutical companies, research institutions, and healthcare facilities, such as the Northeast, Mid-Atlantic, and West Coast, represent key markets for contract manufacturing services. Metropolitan areas with clusters of biopharmaceutical companies, academic centers, and innovation hubs, such as Boston, San Francisco, and New York City, offer opportunities for contract manufacturers to establish a presence and serve local customers.
Competitive Landscape
Leading Companies in US Oral Solid Dosage Contract Manufacturing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The US oral solid dosage contract manufacturing market can be segmented based on various factors, including:
Segmentation provides insights into customer needs, market trends, and growth opportunities, enabling contract manufacturers to tailor their services and solutions to specific market segments and applications.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has underscored the importance of oral solid dosage contract manufacturing in ensuring the availability and accessibility of essential medications, supporting pharmaceutical companies in responding to increased demand, supply chain disruptions, and regulatory challenges. Contract manufacturers have adapted to the pandemic by implementing measures such as enhanced safety protocols, remote working arrangements, and business continuity plans to maintain operations, protect employees, and support pharmaceutical clients in their response efforts.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future outlook for the US oral solid dosage contract manufacturing market is optimistic, driven by factors such as increasing demand for pharmaceutical products, outsourcing trends, technological advancements, and regulatory harmonization efforts. Contract manufacturers are well-positioned to capitalize on market opportunities by offering specialized expertise, quality manufacturing services, and strategic partnerships to pharmaceutical clients seeking cost-effective, scalable solutions for oral solid dosage manufacturing. While challenges such as price competition, quality concerns, and regulatory complexities persist, contract manufacturers can navigate these challenges by focusing on innovation, quality, customer service, and collaboration to sustain growth and success in the dynamic pharmaceutical landscape.
Conclusion
The US oral solid dosage contract manufacturing market plays a vital role in the pharmaceutical industry, providing outsourcing services for the production of tablets, capsules, and other solid dosage forms. Contract manufacturers offer pharmaceutical companies expertise, capacity, and regulatory compliance to accelerate product development, optimize supply chains, and reduce manufacturing costs. While the market faces challenges such as price competition, quality concerns, and regulatory complexities, opportunities for growth exist in biopharmaceuticals, specialty medications, and advanced manufacturing technologies. By focusing on innovation, quality, compliance, and customer service, contract manufacturers can navigate market dynamics, meet customer needs, and drive success in the US oral solid dosage contract manufacturing market.
What is Oral Solid Dosage Contract Manufacturing?
Oral Solid Dosage Contract Manufacturing refers to the production of solid forms of medication, such as tablets and capsules, by third-party manufacturers. This process allows pharmaceutical companies to outsource their manufacturing needs while ensuring compliance with regulatory standards and quality control.
What are the key players in the US Oral Solid Dosage Contract Manufacturing Market?
Key players in the US Oral Solid Dosage Contract Manufacturing Market include Catalent, Lonza, and Aenova, among others. These companies specialize in providing comprehensive manufacturing services, including formulation development and packaging solutions.
What are the growth factors driving the US Oral Solid Dosage Contract Manufacturing Market?
The growth of the US Oral Solid Dosage Contract Manufacturing Market is driven by the increasing demand for generic drugs, the rise in chronic diseases requiring long-term medication, and the need for cost-effective manufacturing solutions. Additionally, advancements in technology and production processes are enhancing efficiency.
What challenges does the US Oral Solid Dosage Contract Manufacturing Market face?
Challenges in the US Oral Solid Dosage Contract Manufacturing Market include stringent regulatory requirements, the complexity of maintaining quality control, and the need for continuous innovation to meet evolving consumer demands. These factors can impact production timelines and costs.
What opportunities exist in the US Oral Solid Dosage Contract Manufacturing Market?
Opportunities in the US Oral Solid Dosage Contract Manufacturing Market include the expansion of personalized medicine, the growing trend of outsourcing manufacturing processes, and the potential for partnerships between pharmaceutical companies and contract manufacturers. These trends can lead to enhanced service offerings and market growth.
What trends are shaping the US Oral Solid Dosage Contract Manufacturing Market?
Trends shaping the US Oral Solid Dosage Contract Manufacturing Market include the increasing adoption of automation in manufacturing processes, the focus on sustainability in production practices, and the rise of digital technologies for better supply chain management. These trends are expected to improve efficiency and reduce environmental impact.
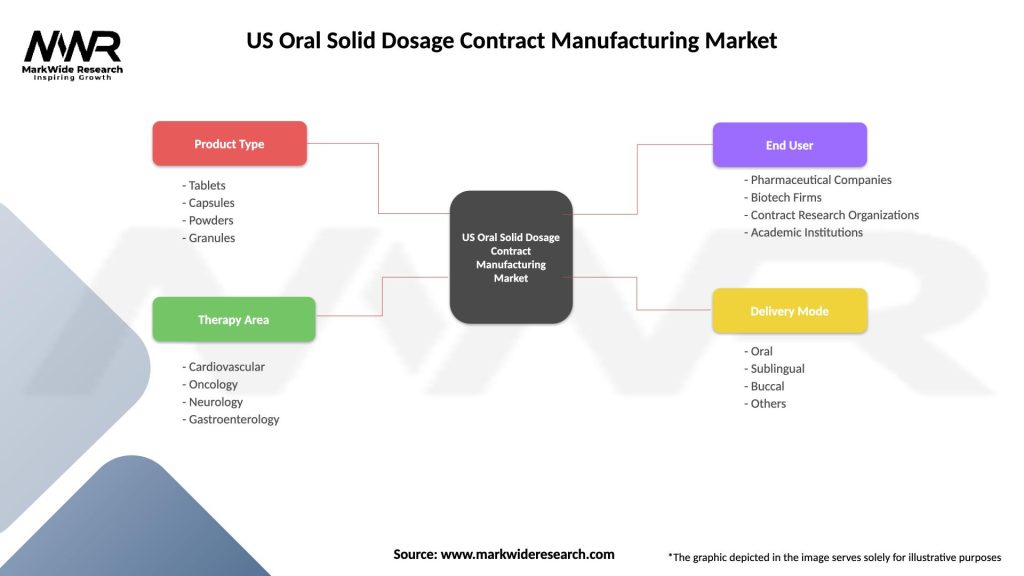
US Oral Solid Dosage Contract Manufacturing Market
| Segmentation Details | Description |
|---|---|
| Product Type | Tablets, Capsules, Powders, Granules |
| Therapy Area | Cardiovascular, Oncology, Neurology, Gastroenterology |
| End User | Pharmaceutical Companies, Biotech Firms, Contract Research Organizations, Academic Institutions |
| Delivery Mode | Oral, Sublingual, Buccal, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in US Oral Solid Dosage Contract Manufacturing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at