444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview
The LAMEA (Latin America, Middle East, and Africa) Bronchial Thermoplasty Catheter Market represents a niche yet vital segment within the medical device industry, catering to the treatment of severe asthma patients who do not respond well to traditional therapies. Bronchial thermoplasty involves the use of specialized catheters to deliver controlled thermal energy to the airway walls, reducing smooth muscle mass and improving asthma symptoms. This innovative treatment option offers hope for patients with severe asthma, addressing unmet medical needs and improving quality of life.
Meaning
Bronchial thermoplasty catheters are medical devices designed for use in bronchial thermoplasty procedures, a minimally invasive treatment for severe asthma. These catheters deliver controlled thermal energy to the airway walls, reducing smooth muscle hypertrophy and airway hyperresponsiveness associated with severe asthma. Bronchial thermoplasty aims to improve asthma control, reduce exacerbations, and enhance patients’ overall quality of life by providing long-term symptom relief and reducing reliance on oral corticosteroids and rescue medications.
Executive Summary
The LAMEA Bronchial Thermoplasty Catheter Market is witnessing steady growth, driven by factors such as increasing prevalence of severe asthma, rising awareness about bronchial thermoplasty as an alternative treatment option, and expanding access to advanced medical technologies in Latin America, the Middle East, and Africa. While challenges such as limited healthcare infrastructure and reimbursement barriers exist, the market offers significant opportunities for growth and innovation in addressing the unmet needs of severe asthma patients in the region.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Key insights into the LAMEA Bronchial Thermoplasty Catheter Market include:
Market Drivers
The growth of the LAMEA Bronchial Thermoplasty Catheter Market is driven by several factors:
Market Restraints
However, the LAMEA Bronchial Thermoplasty Catheter Market faces certain constraints:
Market Opportunities
Despite these challenges, the LAMEA Bronchial Thermoplasty Catheter Market presents opportunities for growth and expansion:
Market Dynamics
The LAMEA Bronchial Thermoplasty Catheter Market operates within a dynamic ecosystem influenced by factors such as demographic trends, healthcare expenditure, regulatory policies, technological innovations, and patient advocacy, shaping market dynamics, adoption rates, and business strategies across the medical device value chain. Understanding these dynamics is essential for stakeholders to identify opportunities, mitigate risks, and navigate market complexities in delivering innovative and effective bronchial thermoplasty solutions to severe asthma patients in Latin America, the Middle East, and Africa.
Regional Analysis
The LAMEA Bronchial Thermoplasty Catheter Market comprises diverse markets, healthcare systems, and regulatory frameworks across Latin America, the Middle East, and Africa. Regional variations in disease burden, healthcare infrastructure, reimbursement policies, and patient demographics influence market dynamics, adoption rates, and commercialization strategies for bronchial thermoplasty catheters in the LAMEA region.
Competitive Landscape
Leading Companies in LAMEA Bronchial Thermoplasty Catheter Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
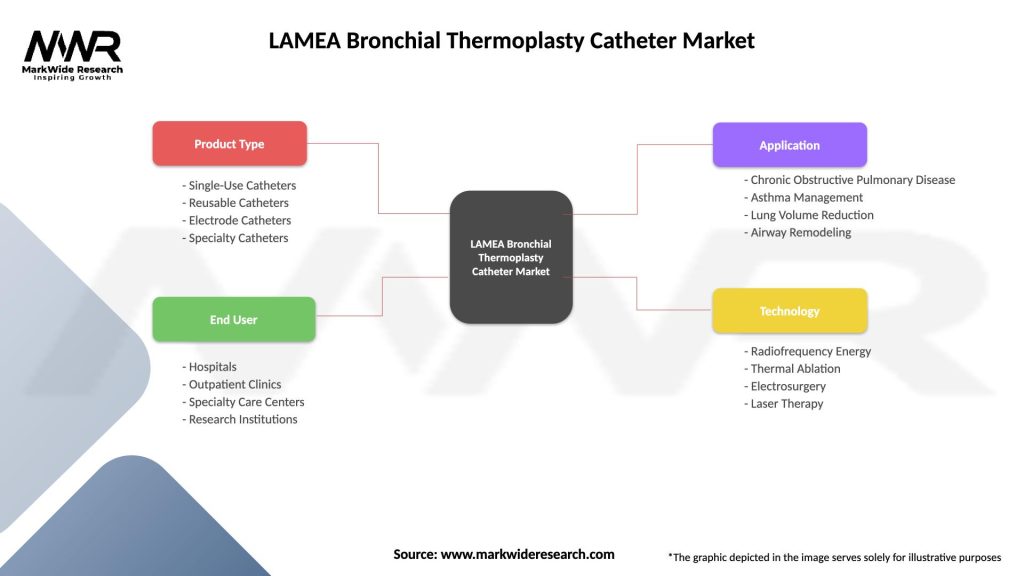
Segmentation
The LAMEA Bronchial Thermoplasty Catheter Market can be segmented based on various factors such as:
Category-wise Insights
From single-use disposable catheters for bronchial thermoplasty procedures to reusable catheters with advanced features for precise energy delivery, each category within the LAMEA Bronchial Thermoplasty Catheter Market offers unique insights into product specifications, clinical performance, and market dynamics shaping adoption rates and patient outcomes across diverse healthcare settings in Latin America, the Middle East, and Africa.
Key Benefits for Industry Participants and Stakeholders
The LAMEA Bronchial Thermoplasty Catheter Market offers several benefits for industry participants and stakeholders, including medical device manufacturers, healthcare providers, regulatory authorities, and patients, such as:
SWOT Analysis
A SWOT analysis of the LAMEA Bronchial Thermoplasty Catheter Market reveals its strengths in clinical efficacy, market expansion, and patient access, alongside weaknesses related to regulatory challenges, reimbursement barriers, and healthcare infrastructure gaps. Opportunities for market growth include technological innovations, market expansion strategies, and patient advocacy initiatives, while threats stem from competition, regulatory uncertainties, and economic constraints. Understanding these factors is essential for stakeholders to develop effective strategies, mitigate risks, and capitalize on market opportunities in the LAMEA Bronchial Thermoplasty Catheter Market.
Market Key Trends
Key trends shaping the LAMEA Bronchial Thermoplasty Catheter Market include:
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the LAMEA Bronchial Thermoplasty Catheter Market, disrupting healthcare services, reducing procedural volumes, and shifting patient priorities and preferences in Latin America, the Middle East, and Africa. While the pandemic has posed short-term challenges such as supply chain disruptions, market access barriers, and financial constraints, it has also accelerated digital health adoption, telemedicine utilization, and virtual patient engagement, driving opportunities for remote patient monitoring, home-based care, and tele-procedural support in the LAMEA region.
Key Industry Developments
Industry developments in the LAMEA Bronchial Thermoplasty Catheter Market include investments in research and development, clinical evidence generation, physician training, and patient education to advance procedural safety, efficacy, and adoption rates in Latin America, the Middle East, and Africa. Collaborative efforts among medical device manufacturers, healthcare providers, regulatory authorities, and patient advocacy groups drive innovation, market access, and patient access to bronchial thermoplasty procedures, addressing unmet medical needs and improving outcomes for severe asthma patients in the region.
Analyst Suggestions
To navigate market uncertainties and capitalize on emerging opportunities in the LAMEA Bronchial Thermoplasty Catheter Market, analysts recommend focusing on innovation, market expansion, patient advocacy, and regulatory compliance to differentiate offerings, drive adoption, and improve patient access and outcomes in Latin America, the Middle East, and Africa.
Future Outlook
The future outlook for the LAMEA Bronchial Thermoplasty Catheter Market is optimistic, with continued growth expected in response to increasing demand for alternative treatment options for severe asthma patients, technological advancements, regulatory harmonization efforts, and patient advocacy initiatives in Latin America, the Middle East, and Africa. However, addressing challenges related to healthcare infrastructure, reimbursement policies, and regulatory compliance will be critical to unlocking the market’s full potential and ensuring long-term success in delivering innovative bronchial thermoplasty solutions to severe asthma patients in the LAMEA region.
Conclusion
In conclusion, the LAMEA Bronchial Thermoplasty Catheter Market represents a dynamic and growing segment within the medical device industry, offering a promising treatment option for severe asthma patients in Latin America, the Middle East, and Africa. Despite challenges such as limited healthcare infrastructure, reimbursement barriers, and regulatory complexities, the market presents significant opportunities for growth and innovation in addressing the unmet medical needs of severe asthma patients in the region. By leveraging technological advancements, market expansion strategies, patient advocacy initiatives, and regulatory compliance efforts, stakeholders can navigate market dynamics, capitalize on emerging opportunities, and improve patient access and outcomes in the LAMEA Bronchial Thermoplasty Catheter Market.
What is Bronchial Thermoplasty Catheter?
Bronchial Thermoplasty Catheter is a medical device used in a procedure aimed at treating severe asthma by reducing the amount of smooth muscle in the airways, thereby decreasing the frequency and severity of asthma attacks.
What are the key players in the LAMEA Bronchial Thermoplasty Catheter Market?
Key players in the LAMEA Bronchial Thermoplasty Catheter Market include Boston Scientific Corporation, Medtronic, and Olympus Corporation, among others.
What are the growth factors driving the LAMEA Bronchial Thermoplasty Catheter Market?
The growth of the LAMEA Bronchial Thermoplasty Catheter Market is driven by the increasing prevalence of asthma, advancements in medical technology, and a growing awareness of innovative treatment options among healthcare providers.
What challenges does the LAMEA Bronchial Thermoplasty Catheter Market face?
Challenges in the LAMEA Bronchial Thermoplasty Catheter Market include regulatory hurdles, high costs associated with the procedure, and limited access to specialized healthcare facilities in certain regions.
What opportunities exist in the LAMEA Bronchial Thermoplasty Catheter Market?
Opportunities in the LAMEA Bronchial Thermoplasty Catheter Market include expanding healthcare infrastructure, increasing investment in respiratory therapies, and the potential for new product innovations to enhance treatment efficacy.
What trends are shaping the LAMEA Bronchial Thermoplasty Catheter Market?
Trends in the LAMEA Bronchial Thermoplasty Catheter Market include the rise of minimally invasive procedures, the integration of digital health technologies, and a focus on personalized medicine to improve patient outcomes.
LAMEA Bronchial Thermoplasty Catheter Market
| Segmentation Details | Description |
|---|---|
| Product Type | Single-Use Catheters, Reusable Catheters, Electrode Catheters, Specialty Catheters |
| End User | Hospitals, Outpatient Clinics, Specialty Care Centers, Research Institutions |
| Application | Chronic Obstructive Pulmonary Disease, Asthma Management, Lung Volume Reduction, Airway Remodeling |
| Technology | Radiofrequency Energy, Thermal Ablation, Electrosurgery, Laser Therapy |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in LAMEA Bronchial Thermoplasty Catheter Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at