444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview
The North America Sarcoma Drugs Market is a critical segment within the pharmaceutical industry, dedicated to addressing the treatment needs of patients diagnosed with sarcomas. Sarcomas are a diverse group of rare cancers that originate in the body’s connective tissues, including bones, muscles, fat, blood vessels, and other soft tissues. The North America Sarcoma Drugs Market encompasses a range of therapeutic interventions, including chemotherapy, targeted therapy, immunotherapy, and emerging treatments such as gene therapy and precision medicine. This market plays a vital role in improving patient outcomes, advancing medical research, and driving innovation in oncology.
Meaning
The North America Sarcoma Drugs Market focuses on the development, manufacturing, and distribution of pharmaceutical products specifically designed to treat sarcomas. Sarcomas are rare and heterogeneous tumors that require specialized treatment approaches tailored to individual patient characteristics, tumor biology, and disease stage. Sarcoma drugs may target specific genetic mutations, signaling pathways, or immune mechanisms involved in tumor growth and progression. The North America Sarcoma Drugs Market aims to provide patients with access to effective and personalized therapies that can improve survival rates, reduce disease progression, and enhance quality of life.
Executive Summary
The North America Sarcoma Drugs Market is characterized by a growing demand for innovative therapies, driven by factors such as increasing incidence of sarcomas, advancements in molecular diagnostics, and expanding treatment options. Despite the challenges posed by the rarity and complexity of sarcomas, pharmaceutical companies, research institutions, and healthcare providers are collaborating to develop novel drugs, conduct clinical trials, and improve patient care. The market offers significant opportunities for drug developers to address unmet medical needs, leverage emerging technologies, and make meaningful contributions to cancer treatment.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The North America Sarcoma Drugs Market operates within a dynamic and evolving landscape shaped by scientific advancements, regulatory developments, market forces, and patient advocacy efforts. Market dynamics influence drug discovery, development, commercialization, and access, driving innovation, collaboration, and competition among stakeholders in the pharmaceutical industry. Understanding the interplay of market dynamics is essential for navigating challenges, seizing opportunities, and advancing the treatment paradigm for sarcomas in North America.
Regional Analysis
The North America Sarcoma Drugs Market encompasses the United States (U.S.) and Canada, two leading economies with advanced healthcare systems, robust research infrastructure, and supportive regulatory frameworks for drug development. The U.S. dominates the North America Sarcoma Drugs Market, accounting for the majority of research funding, clinical trial activity, and pharmaceutical innovation in sarcoma treatment. Canada also contributes to the North America Sarcoma Drugs Market through academic research, clinical collaborations, and patient advocacy initiatives. Collaborations between academic institutions, industry partners, and government agencies drive translational research, drug discovery, and clinical development efforts in North America, positioning the region as a key hub for sarcoma research and drug development globally.
Competitive Landscape
Leading Companies in the North America Sarcoma Drugs Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
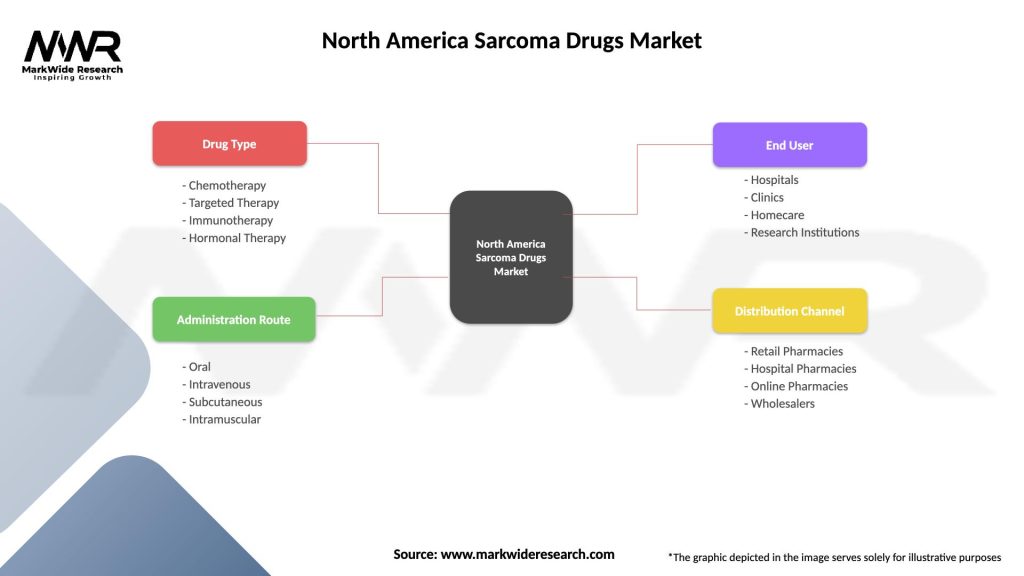
Segmentation
The North America Sarcoma Drugs Market can be segmented based on various factors, including:
Segmentation enables a deeper understanding of market dynamics, patient needs, and therapeutic opportunities within the North America Sarcoma Drugs Market, guiding drug development strategies, clinical trial design, and commercialization efforts.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Understanding these factors through a SWOT analysis helps stakeholders navigate challenges, capitalize on opportunities, and formulate strategies to drive innovation and growth in the North America Sarcoma Drugs Market.
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the North America Sarcoma Drugs Market, disrupting healthcare systems, clinical research activities, and patient care delivery. Key impacts of COVID-19 on the market include:
Key Industry Developments
Analyst Suggestions
Future Outlook
The North America Sarcoma Drugs Market is expected to witness continued growth and innovation, driven by advancements in precision medicine, immunotherapy, targeted therapy, and combination approaches. Despite challenges posed by the COVID-19 pandemic, regulatory complexities, and market dynamics, the sarcoma drug development pipeline remains robust, with promising candidates in various stages of clinical development. The future outlook for the North America Sarcoma Drugs Market is characterized by opportunities for scientific discovery, clinical translation, and patient-centered care delivery, positioning the region as a leader in sarcoma research and treatment globally.
Conclusion
The North America Sarcoma Drugs Market plays a pivotal role in addressing the unmet medical needs of patients diagnosed with sarcomas, rare and heterogeneous tumors with limited treatment options. Despite challenges such as clinical heterogeneity, drug resistance, and regulatory uncertainties, the market offers opportunities for innovation, collaboration, and patient-centric care delivery. By leveraging advancements in precision medicine, immunotherapy, and targeted therapy, stakeholders in the North America Sarcoma Drugs Market can accelerate drug discovery, improve treatment outcomes, and ultimately transform the landscape of sarcoma treatment for patients across the region.
What is Sarcoma Drugs?
Sarcoma drugs are medications specifically designed to treat sarcomas, which are a type of cancer that arises from connective tissues such as bone, fat, muscle, and blood vessels. These drugs can include chemotherapy agents, targeted therapies, and immunotherapies that aim to manage or eliminate sarcoma tumors.
What are the key players in the North America Sarcoma Drugs Market?
Key players in the North America Sarcoma Drugs Market include Pfizer, Eli Lilly, and Novartis, which are known for their contributions to oncology and the development of sarcoma treatments. These companies focus on innovative therapies and clinical trials to enhance treatment options for patients, among others.
What are the growth factors driving the North America Sarcoma Drugs Market?
The North America Sarcoma Drugs Market is driven by factors such as increasing incidence rates of sarcomas, advancements in drug development, and a growing focus on personalized medicine. Additionally, rising awareness and improved diagnostic techniques contribute to market growth.
What challenges does the North America Sarcoma Drugs Market face?
Challenges in the North America Sarcoma Drugs Market include high treatment costs, limited availability of effective therapies for certain sarcoma subtypes, and regulatory hurdles in drug approval processes. These factors can hinder patient access to necessary treatments.
What opportunities exist in the North America Sarcoma Drugs Market?
Opportunities in the North America Sarcoma Drugs Market include the potential for novel drug development, increased investment in research and development, and collaborations between pharmaceutical companies and research institutions. These factors can lead to the introduction of more effective therapies.
What trends are shaping the North America Sarcoma Drugs Market?
Trends in the North America Sarcoma Drugs Market include the rise of targeted therapies and immunotherapies, as well as the integration of biomarker testing in treatment plans. Additionally, there is a growing emphasis on patient-centric approaches and the use of real-world evidence in clinical decision-making.
North America Sarcoma Drugs Market
| Segmentation Details | Description |
|---|---|
| Drug Type | Chemotherapy, Targeted Therapy, Immunotherapy, Hormonal Therapy |
| Administration Route | Oral, Intravenous, Subcutaneous, Intramuscular |
| End User | Hospitals, Clinics, Homecare, Research Institutions |
| Distribution Channel | Retail Pharmacies, Hospital Pharmacies, Online Pharmacies, Wholesalers |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the North America Sarcoma Drugs Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at