444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview:
The Europe intrauterine devices (IUD) market plays a pivotal role in women’s reproductive healthcare, offering a highly effective and long-term contraceptive option. Intrauterine devices are small, T-shaped devices inserted into the uterus to prevent pregnancy by altering the uterine environment. The market encompasses a range of IUD options, providing women with choices that align with their preferences and healthcare needs.
Meaning:
Intrauterine devices, commonly known as IUDs, are contraceptive devices placed inside the uterus to prevent pregnancy. They are available in hormonal and non-hormonal variants and offer a reliable and reversible method of birth control. IUDs are considered a cost-effective and convenient choice, making them a popular option among women seeking long-term contraception.
Executive Summary:
The Europe intrauterine devices market is characterized by a commitment to reproductive health and family planning. As a region with diverse healthcare systems and cultural considerations, the market is shaped by factors such as accessibility, awareness, and regulatory guidelines. The executive summary highlights the market’s steady growth, driven by the efficacy and acceptance of IUDs as a preferred contraceptive method.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The Europe intrauterine devices market operates within a dynamic landscape shaped by factors such as changing demographics, healthcare policies, and evolving societal attitudes towards family planning. These dynamics influence market trends, consumer preferences, and the overall trajectory of contraceptive choices in the region.
Regional Analysis:
Europe exhibits diversity in contraceptive preferences and family planning practices across countries. Factors such as cultural norms, healthcare infrastructure, and economic conditions contribute to variations in the adoption of intrauterine devices. While some countries have embraced IUDs as a mainstream contraceptive option, others may witness a gradual shift in attitudes and acceptance.
Competitive Landscape:
Leading Companies in the Europe Intrauterine Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
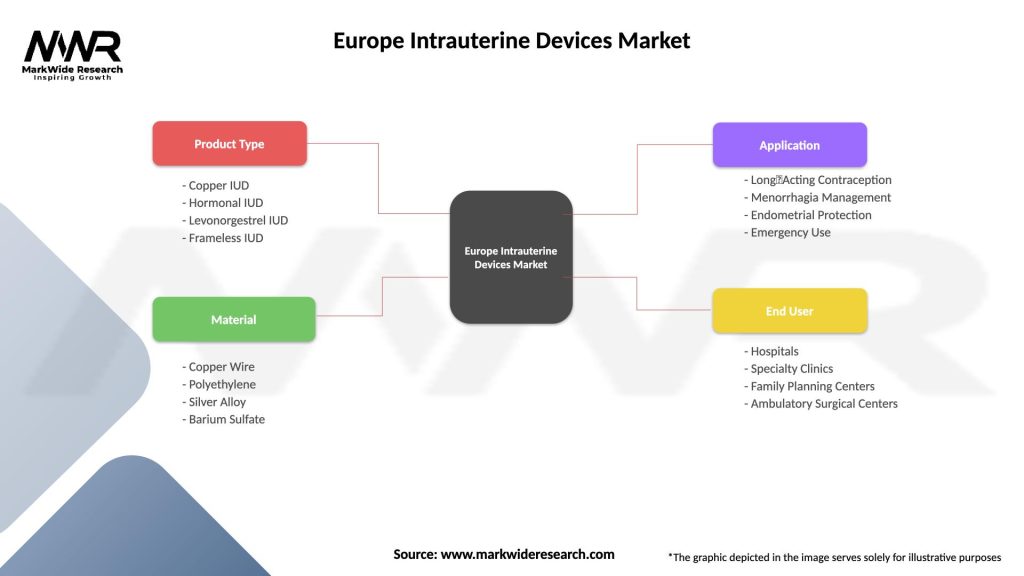
Segmentation:
The Europe intrauterine devices market can be segmented based on factors such as the type of IUD (hormonal or non-hormonal), duration of effectiveness, and geographic considerations. Hormonal IUDs may be preferred by women seeking additional benefits such as menstrual regulation, while non-hormonal options cater to those desiring contraception without hormonal influence.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
A SWOT analysis provides a comprehensive understanding of the Europe intrauterine devices market:
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic has influenced healthcare-seeking behaviors and access to reproductive health services. While the pandemic initially led to disruptions in routine healthcare services, including family planning, the resilience of the intrauterine devices market is evident in its recovery. Efforts to restore and enhance access to contraceptives, coupled with the adaptability of healthcare systems, have contributed to maintaining the market’s stability.
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The Europe intrauterine devices market is poised for continued growth, driven by evolving societal attitudes towards contraception, advancements in technology, and a focus on women’s reproductive health. The industry’s future will be shaped by ongoing innovations, digital health integration, and collaborative efforts to enhance access and awareness.
Conclusion:
The Europe intrauterine devices market occupies a vital position in the region’s reproductive healthcare landscape, offering women a range of contraceptive options aligned with their preferences and health needs. As the market continues to evolve, addressing barriers to adoption, enhancing user experience, and leveraging digital health solutions will be instrumental in ensuring the sustained growth and acceptance of intrauterine devices. By aligning with changing healthcare dynamics, manufacturers, healthcare providers, and stakeholders can contribute to advancing family planning and women’s reproductive autonomy in Europe.
What is Intrauterine Devices?
Intrauterine Devices (IUDs) are small, T-shaped devices inserted into the uterus to prevent pregnancy. They can be hormonal or copper-based and are known for their long-term effectiveness and convenience.
What are the key players in the Europe Intrauterine Devices Market?
Key players in the Europe Intrauterine Devices Market include Bayer AG, CooperSurgical, and Merck KGaA, among others. These companies are involved in the development and distribution of various IUD products across the region.
What are the growth factors driving the Europe Intrauterine Devices Market?
The Europe Intrauterine Devices Market is driven by increasing awareness of long-term contraceptive options, rising demand for family planning, and advancements in IUD technology. Additionally, government initiatives promoting reproductive health contribute to market growth.
What challenges does the Europe Intrauterine Devices Market face?
Challenges in the Europe Intrauterine Devices Market include potential side effects associated with IUDs, cultural resistance to contraceptive methods, and regulatory hurdles in product approval. These factors can impact adoption rates and market penetration.
What opportunities exist in the Europe Intrauterine Devices Market?
Opportunities in the Europe Intrauterine Devices Market include the introduction of innovative IUD designs, increasing investment in women’s health initiatives, and expanding access to reproductive health services. These factors can enhance market potential and consumer acceptance.
What trends are shaping the Europe Intrauterine Devices Market?
Trends in the Europe Intrauterine Devices Market include a growing preference for non-hormonal contraceptive options, increased focus on personalized healthcare, and the integration of digital health technologies in reproductive health management. These trends are influencing consumer choices and market dynamics.
Europe Intrauterine Devices Market
| Segmentation Details | Description |
|---|---|
| Product Type | Copper IUD, Hormonal IUD, Levonorgestrel IUD, Frameless IUD |
| Material | Copper Wire, Polyethylene, Silver Alloy, Barium Sulfate |
| Application | Long‑Acting Contraception, Menorrhagia Management, Endometrial Protection, Emergency Use |
| End User | Hospitals, Specialty Clinics, Family Planning Centers, Ambulatory Surgical Centers |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Europe Intrauterine Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at