444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Medical Device Complaint Management market is a vital component of the healthcare industry that focuses on managing and addressing complaints related to medical devices. These complaints can arise from various stakeholders, including healthcare professionals, patients, and regulatory bodies. Effective complaint management plays a crucial role in ensuring patient safety, regulatory compliance, and overall product quality.
Meaning
Medical device complaint management refers to the systematic process of receiving, evaluating, and addressing complaints associated with medical devices. It involves gathering information about the complaint, investigating its root cause, implementing corrective actions, and communicating with the relevant parties involved. The goal is to resolve the complaint promptly and effectively while maintaining the highest standards of patient safety and product quality.
Executive Summary
The Medical Device Complaint Management market is witnessing significant growth due to the increasing complexity and diversity of medical devices, stringent regulatory requirements, and the growing emphasis on patient safety. This market encompasses various solutions, including complaint handling software, customer support services, and training programs, among others. These solutions help manufacturers, healthcare providers, and regulatory authorities streamline the complaint management process and ensure compliance with applicable regulations.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Medical Device Complaint Management market is driven by various dynamic factors, including technological advancements, regulatory requirements, and the focus on patient safety. These dynamics influence the market’s growth, adoption of innovative solutions, and the competitive landscape.
Regional Analysis
The Medical Device Complaint Management market exhibits regional variations influenced by factors such as regulatory frameworks, healthcare infrastructure, and market maturity. North America and Europe, known for their stringent regulatory environments, have well-established complaint management systems. Emerging markets in Asia Pacific and Latin America are witnessing increasing adoption of complaint management solutions, driven by rising healthcare investments and regulatory developments.
Competitive Landscape
Leading Companies in the Medical Device Complaint Management Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

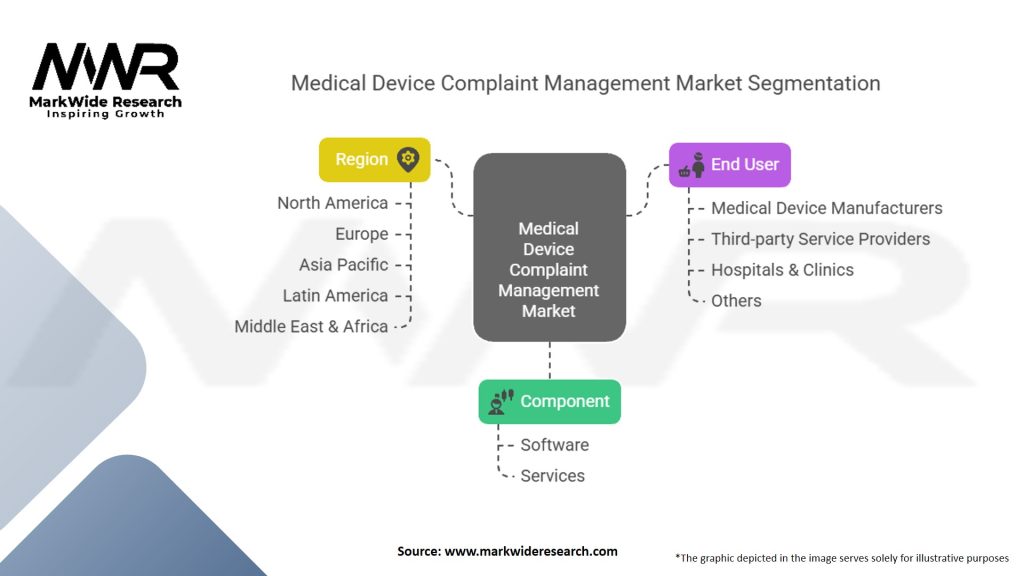
Segmentation
The Medical Device Complaint Management market can be segmented based on various parameters, including:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
A SWOT analysis of the Medical Device Complaint Management market provides a comprehensive assessment of its strengths, weaknesses, opportunities, and threats:
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has significantly impacted the Medical Device Complaint Management market. The pandemic brought to light the importance of effective complaint management in ensuring patient safety and managing device-related issues. The increased focus on medical device quality and safety, driven by the pandemic, has further emphasized the need for streamlined complaint management processes and advanced solutions.
The pandemic has also accelerated the adoption of digital technologies in complaint management. Remote work arrangements and limited access to physical facilities have necessitated the use of cloud-based complaint handling systems and virtual communication channels. These changes are likely to continue even after the pandemic, as organizations recognize the benefits of remote accessibility and flexibility.
However, the pandemic has also posed challenges, such as disruptions in supply chains, delays in complaint resolution due to limited resources, and increased cybersecurity risks. Market players have had to adapt quickly to these challenges, implementing contingency plans and ensuring business continuity.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the Medical Device Complaint Management market looks promising, driven by advancements in technology, increasing regulatory scrutiny, and the growing focus on patient safety. The integration of AI, ML, and data analytics will continue to transform complaint management processes, enabling faster complaint resolution and proactive issue identification.
The market is expected to witness further consolidation, with established players expanding their product portfolios through collaborations, partnerships, and acquisitions. The emphasis on regulatory compliance and post-market surveillance will drive organizations to invest in comprehensive complaint management solutions.
The Covid-19 pandemic has accelerated the digital transformation of complaint management, and these changes are likely to persist in the future. The industry will continue to adapt to evolving regulatory requirements, market dynamics, and technological advancements to ensure efficient and effective complaint management processes.
Conclusion
The Medical Device Complaint Management market plays a critical role in ensuring patient safety, regulatory compliance, and product quality. The increasing complexity of medical devices, stringent regulatory requirements, and the focus on patient safety are driving the adoption of advanced complaint management solutions.
What is Medical Device Complaint Management?
Medical Device Complaint Management refers to the processes and systems used to handle complaints related to medical devices, ensuring compliance with regulatory standards and improving product safety. It involves tracking, investigating, and resolving issues reported by users or healthcare professionals.
What are the key players in the Medical Device Complaint Management Market?
Key players in the Medical Device Complaint Management Market include Medtronic, Boston Scientific, and Johnson & Johnson, among others. These companies are involved in developing robust complaint management systems to enhance product quality and safety.
What are the main drivers of growth in the Medical Device Complaint Management Market?
The growth of the Medical Device Complaint Management Market is driven by increasing regulatory requirements, the rising number of medical device recalls, and the growing emphasis on patient safety. Additionally, advancements in technology are facilitating better complaint tracking and resolution.
What challenges does the Medical Device Complaint Management Market face?
Challenges in the Medical Device Complaint Management Market include the complexity of regulatory compliance, the need for effective data management systems, and the potential for underreporting of complaints. These factors can hinder the efficiency of complaint resolution processes.
What opportunities exist in the Medical Device Complaint Management Market?
Opportunities in the Medical Device Complaint Management Market include the integration of artificial intelligence for data analysis, the development of more user-friendly complaint reporting systems, and the expansion of services to emerging markets. These innovations can enhance overall complaint management efficiency.
What trends are shaping the Medical Device Complaint Management Market?
Trends in the Medical Device Complaint Management Market include the increasing use of digital platforms for complaint reporting, a focus on proactive risk management, and the adoption of real-time monitoring systems. These trends aim to improve responsiveness and enhance patient safety.
Medical Device Complaint Management Market
| Segmentation | Details |
|---|---|
| Component | Software, Services (Consulting, Implementation, Support) |
| End User | Medical Device Manufacturers, Third-party Service Providers, Hospitals & Clinics, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Medical Device Complaint Management Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at