444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview
The US digital therapeutics market is witnessing significant growth, driven by the increasing adoption of digital technologies in healthcare. Digital therapeutics, also known as software-based therapeutics or digital health interventions, refer to evidence-based therapeutic interventions that utilize digital technologies to prevent, manage, or treat medical conditions. These interventions are designed to deliver personalized healthcare through the use of software applications, mobile devices, wearables, and other digital platforms.
Meaning
Digital therapeutics represent a paradigm shift in the healthcare industry, offering new opportunities for patient care and management. Unlike traditional pharmaceutical treatments, digital therapeutics focus on delivering targeted interventions that can be customized to the individual needs of patients. These interventions can range from mobile apps that provide cognitive behavioral therapy for mental health conditions to sensor-based devices that monitor and manage chronic diseases such as diabetes or hypertension.
Executive Summary
The US digital therapeutics market has experienced substantial growth in recent years, fueled by factors such as the increasing prevalence of chronic diseases, rising healthcare costs, and advancements in technology. The market is characterized by the presence of numerous players, including established pharmaceutical companies, technology firms, and startups specializing in digital health solutions. These companies are investing heavily in research and development to develop innovative digital therapeutics that can address a wide range of medical conditions.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Several key insights define the US digital therapeutics market. First, the market is witnessing robust growth due to the increasing adoption of smartphones and wearable devices, which serve as platforms for delivering digital therapeutics. The convenience and accessibility offered by these devices make them an ideal medium for patient engagement and adherence to treatment plans.
Second, the market is being driven by the growing emphasis on preventive healthcare and patient-centered care models. Digital therapeutics provide opportunities for early detection, intervention, and personalized treatment plans, helping patients take charge of their health and well-being.
Third, the market is witnessing collaborations and partnerships between pharmaceutical companies and digital health firms to leverage their respective expertise. These partnerships aim to combine the pharmaceutical industry’s regulatory knowledge and distribution networks with the technological prowess of digital health companies.
Market Drivers
Several factors are driving the growth of the US digital therapeutics market. Firstly, the increasing prevalence of chronic diseases, such as diabetes, cardiovascular diseases, and respiratory disorders, is creating a significant demand for digital therapeutics. These interventions offer cost-effective and scalable solutions for managing these chronic conditions and reducing the burden on healthcare systems.
Secondly, the rising healthcare costs and the need for efficient healthcare delivery are compelling healthcare providers and payers to explore innovative solutions. Digital therapeutics provide opportunities for remote monitoring, telehealth consultations, and self-management, enabling cost savings and improved patient outcomes.
Furthermore, advancements in technology, including artificial intelligence, machine learning, and data analytics, are enabling the development of sophisticated digital therapeutics. These technologies enhance the accuracy of diagnosis, personalization of treatment plans, and real-time monitoring of patients, contributing to the market’s growth.
Market Restraints
Despite the positive growth trajectory, the US digital therapeutics market faces certain challenges and restraints. One of the primary challenges is the lack of regulatory clarity and standardized guidelines for digital therapeutics. Unlike pharmaceutical products, digital therapeutics fall into a regulatory gray area, making it difficult for companies to navigate the approval and reimbursement processes.
Another restraint is the limited awareness and acceptance of digital therapeutics among healthcare professionals and patients. Skepticism regarding the effectiveness and reliability of these interventions can hinder their adoption, even though there is a growing body of evidence supporting their efficacy.
Moreover, issues related to data privacy and security pose concerns for the widespread adoption of digital therapeutics. Collecting and transmitting sensitive health data through digital platforms require robust security measures to protect patient information from breaches and unauthorized access.
Market Opportunities
Despite the challenges, the US digital therapeutics market presents significant opportunities for growth and innovation. The integration of digital therapeutics into mainstream healthcare systems can enhance patient outcomes, improve healthcare efficiency, and reduce costs. Healthcare providers and payers can leverage these opportunities to implement value-based care models and drive patient engagement and adherence.
Furthermore, the market offers opportunities for collaboration between technology companies, pharmaceutical manufacturers, and healthcare providers. By combining their expertise and resources, these stakeholders can develop comprehensive digital therapeutics solutions that integrate seamlessly into existing healthcare workflows and delivery models.
Additionally, the increasing focus on mental health and well-being presents a promising avenue for digital therapeutics. The rising prevalence of mental health disorders, coupled with the stigma associated with seeking traditional therapy, creates a demand for digital mental health interventions that provide privacy, accessibility, and personalized support.
Market Dynamics
The US digital therapeutics market is characterized by intense competition and dynamic market dynamics. The market is witnessing a surge in partnerships, collaborations, and mergers and acquisitions as companies aim to strengthen their product portfolios and expand their market presence.
Companies are investing in research and development activities to develop novel digital therapeutics targeting a wide range of therapeutic areas. These interventions encompass various modalities, including cognitive behavioral therapy, medication adherence programs, remote monitoring, and personalized coaching.
Additionally, the market is driven by reimbursement initiatives and regulatory advancements. The Centers for Medicare and Medicaid Services (CMS) and private insurers are exploring reimbursement models for digital therapeutics, recognizing their potential to improve patient outcomes and reduce healthcare costs. Clear regulatory guidelines and frameworks for digital therapeutics will further stimulate market growth and foster innovation.
Regional Analysis
The US digital therapeutics market exhibits regional variations in terms of adoption, awareness, and market maturity. The market is most developed in metropolitan areas with advanced healthcare infrastructure and a high concentration of technology companies. Cities like San Francisco, Boston, and New York serve as hubs for digital health innovation and attract startups and established players alike.
However, there are opportunities for market expansion in rural and underserved areas, where access to specialized healthcare services is limited. Digital therapeutics can bridge the gap by providing remote consultations, virtual care, and self-management tools to patients in these regions.
Furthermore, regional variations exist in terms of regulatory environments and reimbursement policies. State-level initiatives and collaborations between regional healthcare systems and technology companies can drive the adoption of digital therapeutics across different geographies.
Competitive Landscape
Leading Companies in the US Digital Therapeutics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
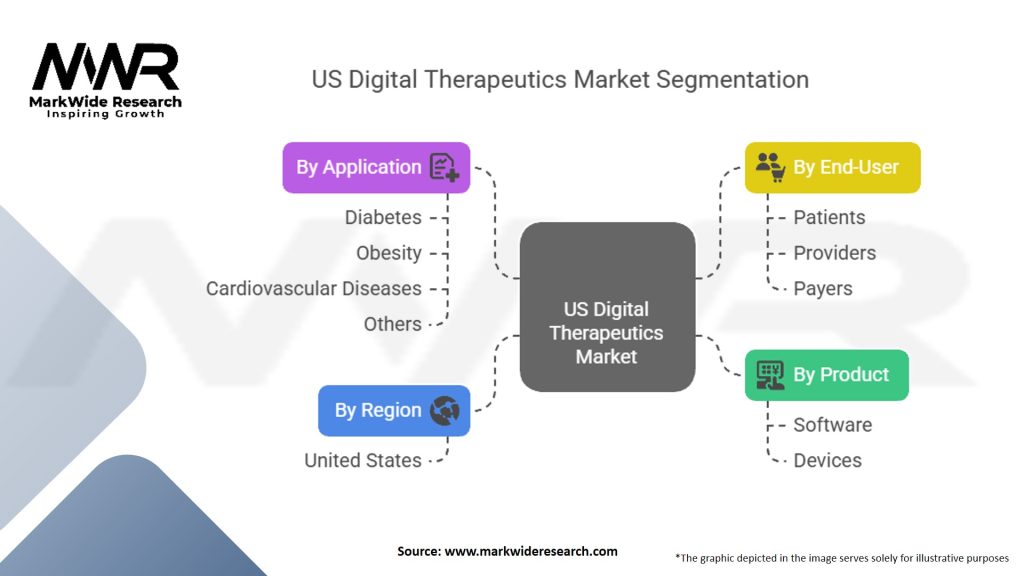
Segmentation
The US digital therapeutics market can be segmented based on therapeutic area, type of intervention, and end-user.
Based on therapeutic area, the market can be categorized into:
Based on the type of intervention, the market can be segmented into:
Based on end-user, the market can be segmented into:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
Industry participants and stakeholders in the US digital therapeutics market can benefit from several key advantages:
SWOT Analysis
The SWOT analysis of the US digital therapeutics market provides a comprehensive overview of its strengths, weaknesses, opportunities, and threats:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Several key trends are shaping the US digital therapeutics market:
Covid-19 Impact
The COVID-19 pandemic has accelerated the adoption of digital therapeutics in the US healthcare system. The pandemic highlighted the importance of remote care delivery, virtual consultations, and self-management tools. Digital therapeutics provided a means to continue healthcare services while minimizing the risk of viral transmission.
The pandemic also highlighted the need for mental health support, as individuals faced increased stress, anxiety, and social isolation. Digital therapeutics for mental health, including virtual counseling and mindfulness apps, saw a surge in demand.
Additionally, the pandemic brought attention to the vulnerability of individuals with chronic conditions. Digital therapeutics for remote monitoring, medication adherence, and self-management became essential tools for managing chronic diseases while reducing the strain on healthcare resources.
The COVID-19 pandemic acted as a catalyst for the widespread adoption of digital therapeutics, further solidifying their role in the future of healthcare delivery.
Key Industry Developments
The US digital therapeutics market has witnessed several key industry developments in recent years:
Analyst Suggestions
Analysts suggest the following strategies for industry participants in the US digital therapeutics market:
Future Outlook
The future outlook for the US digital therapeutics market is highly promising. The market is expected to witness continued growth driven by technological advancements, increasing healthcare costs, and the need for personalized, patient-centric care. The integration of digital therapeutics into mainstream healthcare systems will become more prevalent, with healthcare providers incorporating these interventions into their care delivery models. Remote monitoring, telehealth consultations, and self-management tools will become standard components of healthcare services.
The regulatory landscape is expected to evolve, with clearer guidelines and frameworks for digital therapeutics. Regulatory approvals and reimbursement mechanisms will become more streamlined, facilitating market entry for innovative interventions.
Furthermore, advancements in AI, machine learning, and data analytics will enable the development of more sophisticated and targeted digital therapeutics. Personalized treatment plans, real-time monitoring, and predictive analytics will become integral to these interventions.
The market will witness increased collaboration between pharmaceutical companies, technology firms, and healthcare providers. These collaborations will drive innovation, enhance product portfolios, and improve market penetration.
Conclusion
The US digital therapeutics market is witnessing rapid growth and transformation, driven by advancements in technology, increasing chronic disease burden, and the need for personalized, accessible healthcare solutions. Digital therapeutics offer innovative interventions that leverage digital technologies to prevent, manage, and treat a wide range of medical conditions. Despite the challenges and uncertainties, the market presents numerous opportunities for industry participants and stakeholders. Collaboration, evidence generation, user experience, and addressing regulatory and reimbursement challenges will be crucial for success in this dynamic market. With ongoing research, technological advancements, and supportive regulatory frameworks, digital therapeutics are poised to play a significant role in shaping the future of healthcare, improving patient outcomes, and reducing healthcare costs in the United States.
What are digital therapeutics in the context of the US digital therapeutics market?
Digital therapeutics are evidence-based therapeutic interventions delivered via software to prevent, manage, or treat medical disorders or diseases. They often complement traditional therapies and can be used for conditions such as diabetes, mental health disorders, and substance abuse.
Who are the key players in the US digital therapeutics market?
Key players in the US digital therapeutics market include companies like Omada Health, Pear Therapeutics, and WellDoc, which focus on various therapeutic areas such as chronic disease management and mental health, among others.
What are the main drivers of growth in the US digital therapeutics market?
The growth of the US digital therapeutics market is driven by increasing prevalence of chronic diseases, rising healthcare costs, and a growing acceptance of digital health solutions among patients and providers. Additionally, advancements in technology and data analytics are enhancing the effectiveness of these therapies.
What challenges does the US digital therapeutics market face?
The US digital therapeutics market faces challenges such as regulatory hurdles, concerns over data privacy, and the need for clinical validation of therapeutic claims. Additionally, reimbursement issues can hinder widespread adoption among healthcare providers.
What opportunities exist for innovation in the US digital therapeutics market?
There are significant opportunities for innovation in the US digital therapeutics market, particularly in areas like personalized medicine, integration with wearable devices, and the development of solutions for underserved populations. The increasing focus on mental health and wellness also presents new avenues for growth.
What trends are shaping the US digital therapeutics market?
Trends shaping the US digital therapeutics market include the rise of mobile health applications, increased collaboration between tech companies and healthcare providers, and a shift towards value-based care models. These trends are influencing how digital therapeutics are developed and implemented in clinical settings.
US Digital Therapeutics Market:
| Segmentation Details | Information |
|---|---|
| By Product | Software, Devices |
| By Application | Diabetes, Obesity, Cardiovascular Diseases, Others |
| By End-User | Patients, Providers, Payers |
| By Region | United States |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the US Digital Therapeutics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at