444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Regulatory Affairs market plays a crucial role in ensuring compliance with various regulations and guidelines set by regulatory bodies in different industries. It encompasses a wide range of activities, including obtaining regulatory approvals, managing product registrations, and ensuring compliance with safety and quality standards. The market has witnessed significant growth in recent years, driven by the increasing complexity of regulatory requirements and the need for organizations to navigate through the regulatory landscape efficiently.
Meaning
Regulatory Affairs refers to the function within an organization that is responsible for ensuring compliance with regulations and guidelines set by regulatory authorities. It involves activities such as regulatory strategy development, product registration and approval, labeling and packaging compliance, and post-market surveillance. The primary goal of Regulatory Affairs is to ensure that products and processes meet the necessary regulatory requirements, thereby minimizing risks and ensuring consumer safety.
Executive Summary
The Regulatory Affairs market has experienced steady growth in recent years, driven by the increasing focus on safety and quality standards across industries. Organizations are increasingly realizing the importance of compliance with regulations to gain market access and maintain a competitive edge. The market is characterized by the presence of numerous regulatory bodies and evolving regulatory frameworks, creating a complex landscape for businesses. As a result, there is a growing demand for Regulatory Affairs professionals and solutions that can help navigate through these challenges efficiently.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Regulatory Affairs market is driven by the interplay of various dynamics, including evolving regulatory frameworks, industry trends, technological advancements, and market forces. These dynamics shape the demand for Regulatory Affairs services and solutions and influence the strategies adopted by organizations to ensure compliance and market access.
The increasing complexity of regulatory requirements and the need for specialized expertise drive the demand for Regulatory Affairs professionals and consulting services. Organizations across industries are investing in regulatory technology solutions to streamline their processes, improve efficiency, and ensure compliance.
The globalization of markets and the trend towards harmonization of regulations present both opportunities and challenges for organizations. On one hand, harmonized regulations simplify market entry and reduce compliance complexities. On the other hand, variations in regulations between different jurisdictions require organizations to have a deep understanding of regional requirements and adapt their strategies accordingly.
The COVID-19 pandemic has also had an impact on the Regulatory Affairs market. Regulatory bodies have implemented emergency measures and expedited approval processes to facilitate the development and availability of vaccines, therapeutics, and medical devices. The pandemic has highlighted the importance of regulatory agility and the need for efficient regulatory processes during times of crisis.
Furthermore, advancements in technology are transforming the Regulatory Affairs landscape. Digital solutions, such as electronic submission systems, cloud-based regulatory information management systems, and automated compliance monitoring tools, are gaining traction in the market. These solutions help organizations streamline their regulatory processes, improve data integrity, and enhance compliance.
Regional Analysis
The Regulatory Affairs market is highly influenced by regional variations in regulatory frameworks and industry dynamics. The market dynamics and growth opportunities differ across regions, driven by factors such as the maturity of regulatory systems, industry sophistication, and market size.
North America: The North American market, comprising the United States and Canada, is one of the largest and most mature markets for Regulatory Affairs. The region is characterized by stringent regulatory requirements, particularly in sectors such as pharmaceuticals, medical devices, and food and beverages. The presence of major regulatory bodies, such as the U.S. Food and Drug Administration (FDA), drives the demand for Regulatory Affairs services and solutions. The market in North America is also driven by technological advancements and the presence of key industry players.
Europe: Europe is another significant market for Regulatory Affairs, with countries such as Germany, France, and the United Kingdom playing a prominent role. The European Union (EU) has a well-established regulatory framework and a centralized approval process for pharmaceuticals and medical devices. The market in Europe is driven by the harmonization of regulations across EU member states, advancements in healthcare technology, and the presence of leading pharmaceutical and medical device companies.
Asia-Pacific: The Asia-Pacific region is witnessing rapid growth in the Regulatory Affairs market, driven by the expansion of pharmaceutical, medical device, and food and beverage industries. Countries such as China, India, and Japan are key contributors to the market growth in the region. The market in Asia-Pacific is characterized by evolving regulatory frameworks, increasing adoption of digital solutions, and the presence of a large patient population.
Latin America: Latin America presents significant growth opportunities for the Regulatory Affairs market. The region is characterized by a growing pharmaceutical and medical device industry, increasing regulatory harmonization efforts, and a focus on improving healthcare infrastructure. Countries such as Brazil and Mexico are key markets in Latin America, driven by the presence of major pharmaceutical companies and the need for regulatory compliance.
Competitive Landscape
Leading Companies in the Regulatory Affairs Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

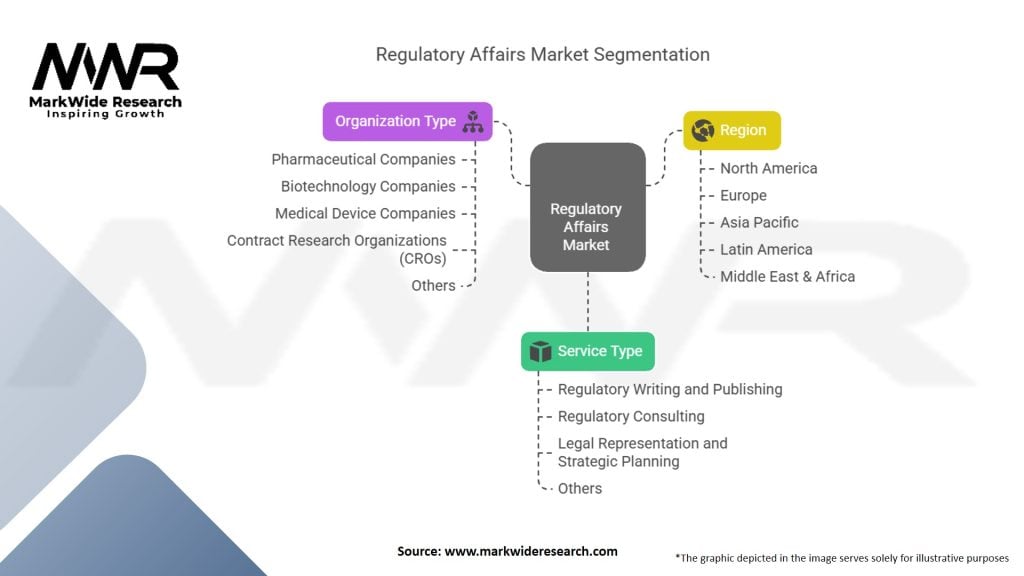
Segmentation
The Regulatory Affairs market can be segmented based on various factors, including industry vertical, service type, and geography.
By Industry Vertical:
By Service Type:
By Geography:
Category-wise Insights
Pharmaceuticals: The pharmaceutical industry is a major segment of the Regulatory Affairs market. Stringent regulations govern the development, manufacturing, and marketing of pharmaceutical products. Regulatory Affairs professionals play a critical role in obtaining product approvals, managing regulatory submissions, and ensuring compliance with Good Manufacturing Practices (GMP) and Good Clinical Practices (GCP). The pharmaceutical industry is witnessing trends such as increased focus on personalized medicine, accelerated approval pathways, and the use of real-world evidence, which present both opportunities and challenges for Regulatory Affairs.
Medical Devices: The medical device industry is another important category within the Regulatory Affairs market. Medical devices, ranging from simple instruments to complex implantable devices, require regulatory approvals before they can be marketed. Regulatory Affairs professionals help navigate the complex regulatory requirements, including product classification, conformity assessment, and post-market surveillance. The increasing innovation and technological advancements in medical devices, such as wearable devices and digital health solutions, create new regulatory challenges and opportunities.
Food and Beverages: The food and beverages industry is subject to strict regulations related to food safety, labeling, and product claims. Regulatory Affairs professionals in this category work closely with food manufacturers, processors, and distributors to ensure compliance with food safety standards and labeling requirements. The market is driven by the increasing demand for transparency in food production, the growing popularity of organic and natural products, and the need for international market access.
Cosmetics and Personal Care: The cosmetics and personal care industry is governed by regulations related to product safety, labeling, and claims. Regulatory Affairs professionals in this category assist companies in ensuring compliance with regulations, such as the European Union Cosmetics Regulation and the U.S. Food, Drug, and Cosmetic Act. The market is influenced by trends such as the growing demand for clean and natural beauty products, the rise of personalized cosmetics, and the increasing importance of sustainability and ethical practices.
Chemicals and Agrochemicals: The chemicals and agrochemicals industry faces extensive regulatory requirements due to the potential risks associated with chemicals and their impact on human health and the environment. Regulatory Affairs professionals help companies navigate through complex regulations, such as REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) in the European Union and the Toxic Substances Control Act (TSCA) in the United States. The market is driven by factors such as the need for sustainable and environmentally friendly chemicals, the increasing demand for agricultural productivity, and the growing focus on chemical safety.
Others: The Regulatory Affairs market also encompasses other industry verticals, such as biotechnology, veterinary products, and tobacco. These industries have unique regulatory requirements and rely on Regulatory Affairs professionals to ensure compliance and market access.
Key Benefits for Industry Participants and Stakeholders
The Regulatory Affairs market offers several key benefits for industry participants and stakeholders, including:
SWOT Analysis
A SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis of the Regulatory Affairs market provides insights into its internal and external factors:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
The Regulatory Affairs market is influenced by several key trends that are shaping the industry:
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the Regulatory Affairs market. The regulatory landscape quickly adapted to facilitate the development, approval, and distribution of vaccines, therapeutics, and medical devices to combat the virus. Regulatory bodies implemented emergency measures, accelerated approval processes, and collaborated with industry stakeholders to ensure timely access to critical products.
The pandemic highlighted the importance of regulatory agility and efficient regulatory processes during times of crisis. Regulatory authorities implemented expedited review procedures, facilitated virtual inspections, and encouraged innovative regulatory approaches to fast-track the development and availability of COVID-19 products.
Furthermore, the pandemic accelerated the adoption of digital solutions in Regulatory Affairs. Electronic submission systems, virtual meetings, and remote inspections became essential tools for regulatory authorities and industry stakeholders to continue their operations while maintaining safety measures.
The COVID-19 pandemic also exposed vulnerabilities in supply chains and regulatory frameworks, leading to discussions on the need for greater resilience and preparedness in the face of future health crises. These discussions are expected to shape future regulatory strategies and practices.
Key Industry Developments
The Regulatory Affairs market has witnessed several key industry developments in recent years:
Analyst Suggestions
Based on the market trends and developments, analysts suggest the following strategies for industry participants in the Regulatory Affairs market:
Future Outlook
The Regulatory Affairs market is expected to continue growing in the coming years, driven by evolving regulatory requirements, technological advancements, and globalization. The increasing complexity of regulations and the need for specialized expertise will drive the demand for Regulatory Affairs professionals and services.
Technological advancements, such as AI, machine learning, and blockchain, will further transform Regulatory Affairs processes, improving efficiency, data integrity, and compliance management. Digital solutions and regulatory technology will become integral to regulatory operations, streamlining processes and enabling real-time monitoring and reporting.
The trend towards global harmonization of regulations is expected to continue, facilitating market access for organizations operating in multiple jurisdictions. However, variations in regulations between different regions will remain a challenge, requiring organizations to have a deep understanding of regional requirements.
Conclusion
In conclusion, the Regulatory Affairs market is poised for growth, driven by increasing regulatory complexities, technological advancements, and the need for compliance and market access. Organizations that prioritize regulatory compliance, invest in specialized expertise, and adopt digital solutions will be well-positioned to navigate the evolving regulatory landscape and gain a competitive advantage.
What is Regulatory Affairs?
Regulatory Affairs refers to the discipline that ensures compliance with regulations and laws governing the development, approval, and marketing of products, particularly in the pharmaceutical, biotechnology, and medical device industries.
Who are the key players in the Regulatory Affairs Market?
Key players in the Regulatory Affairs Market include companies like Parexel International, Covance, and Charles River Laboratories, which provide regulatory consulting and compliance services, among others.
What are the main drivers of growth in the Regulatory Affairs Market?
The main drivers of growth in the Regulatory Affairs Market include the increasing complexity of regulations, the rising demand for new therapies, and the globalization of clinical trials, which necessitate robust regulatory strategies.
What challenges does the Regulatory Affairs Market face?
Challenges in the Regulatory Affairs Market include the evolving regulatory landscape, the need for continuous training and expertise, and the potential for delays in product approvals due to stringent compliance requirements.
What opportunities exist in the Regulatory Affairs Market?
Opportunities in the Regulatory Affairs Market include the expansion of personalized medicine, the growth of digital health technologies, and the increasing focus on regulatory harmonization across regions.
What trends are shaping the Regulatory Affairs Market?
Trends shaping the Regulatory Affairs Market include the adoption of artificial intelligence for regulatory submissions, the emphasis on patient-centric approaches in clinical trials, and the integration of real-world evidence in regulatory decision-making.
Regulatory Affairs Market
| Segmentation Details | Information |
|---|---|
| Service Type | Regulatory Writing and Publishing, Regulatory Consulting, Legal Representation and Strategic Planning, Others |
| Organization Type | Pharmaceutical Companies, Biotechnology Companies, Medical Device Companies, Contract Research Organizations (CROs), Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Regulatory Affairs Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at