444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The global hepatitis E diagnostic tests market is expected to grow at a significant rate in the coming years due to the rising prevalence of hepatitis E virus (HEV) infection worldwide. Hepatitis E is a viral infection that primarily affects the liver and can lead to serious complications if left untreated. It is caused by the hepatitis E virus, which is transmitted through contaminated water or food.
The global hepatitis E diagnostic tests market is expected to witness a compound annual growth rate (CAGR) of approximately 5.2% during the forecast period from 2021 to 2028. The market was valued at USD 63.7 million in 2020 and is expected to reach USD 93.7 million by 2028.
Hepatitis E is a viral infection that affects the liver and is caused by the hepatitis E virus (HEV). The virus is primarily transmitted through contaminated water or food, and is common in developing countries with poor sanitation and hygiene. The symptoms of hepatitis E infection can range from mild to severe, and can include fever, fatigue, nausea, vomiting, abdominal pain, and jaundice.
There are several diagnostic tests available for hepatitis E, including serology tests, nucleic acid amplification tests (NAAT), and enzyme-linked immunosorbent assays (ELISA). These tests can help healthcare professionals diagnose the infection and determine the severity of the disease.
Executive Summary
The global hepatitis E diagnostic tests market is expected to grow at a CAGR of 5.2% during the forecast period from 2021 to 2028. The market is driven by the rising prevalence of hepatitis E infection worldwide, as well as advancements in diagnostic technologies.
Serology tests, NAAT, and ELISA are the most commonly used diagnostic tests for hepatitis E. These tests can help healthcare professionals diagnose the infection and determine the severity of the disease.
North America is the largest market for hepatitis E diagnostic tests, followed by Europe and Asia Pacific. The market is highly competitive, with several key players competing for market share.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
The global hepatitis E diagnostic tests market is expected to grow at a significant rate in the coming years due to the rising prevalence of hepatitis E infection worldwide. Hepatitis E is more common in developing countries with poor sanitation and hygiene, but it can also affect people in developed countries. The increasing awareness about the disease and the need for early diagnosis and treatment are driving the demand for hepatitis E diagnostic tests.
The market is highly competitive, with several key players competing for market share. Some of the major players in the market include MP Biomedicals, BioMérieux SA, and DiaSorin S.p.A. These companies are focusing on product development and innovation to gain a competitive edge in the market.
Serology tests, NAAT, and ELISA are the most commonly used diagnostic tests for hepatitis E. These tests can help healthcare professionals diagnose the infection and determine the severity of the disease. However, these tests have some limitations and may not be accurate in certain situations. For example, serology tests may produce false-positive results in patients with autoimmune diseases, and NAAT may not be able to detect all strains of the virus.
North America is the largest market for hepatitis E diagnostic tests, followed by Europe and Asia Pacific. The high prevalence of hepatitis E in North America, coupled with the presence of several key players in the region, is driving the growth of the market.
Market Analysis
The global hepatitis E diagnostic tests market is expected to witness a CAGR of 5.2% during the forecast period from 2021 to 2028. The market was valued at USD 63.7 million in 2020 and is expected to reach USD 93.7 million by 2028.
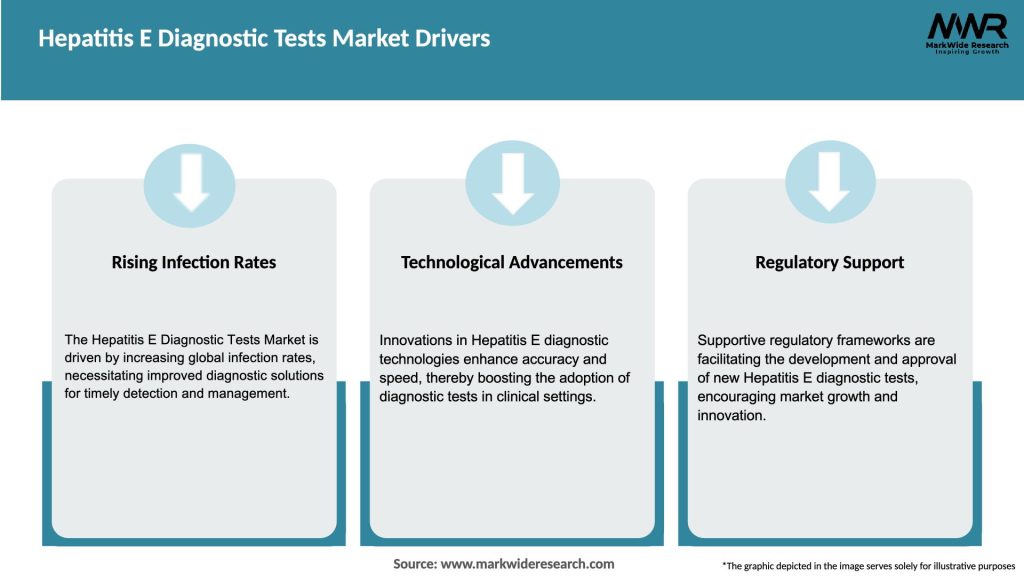
Market Drivers
The rising prevalence of hepatitis E infection worldwide is one of the major drivers of the global hepatitis E diagnostic tests market. According to the World Health Organization (WHO), hepatitis E is responsible for an estimated 20 million infections and 44,000 deaths worldwide each year. The increasing awareness about the disease and the need for early diagnosis and treatment are driving the demand for hepatitis E diagnostic tests.
Advancements in diagnostic technologies are also driving the growth of the market. The development of new and improved diagnostic tests, such as real-time polymerase chain reaction (PCR) and next-generation sequencing (NGS), is providing healthcare professionals with more accurate and reliable tools for diagnosing hepatitis E infection.
Market Restraints
One of the major restraints of the global hepatitis E diagnostic tests market is the high cost of diagnostic tests. In many developing countries, where hepatitis E is most prevalent, the cost of diagnostic tests may be prohibitive for many patients. This is limiting the uptake of diagnostic tests and hindering the growth of the market in these regions.
The limited availability of diagnostic tests in some regions is also a major restraint of the market. In many developing countries, healthcare facilities may not have access to the latest diagnostic technologies, which can hinder the timely diagnosis and treatment of hepatitis E infection.
Market Opportunities
The increasing demand for point-of-care (POC) diagnostic tests is creating new opportunities in the global hepatitis E diagnostic tests market. POC tests are simple, portable, and easy to use, and can provide rapid results in a matter of minutes. This makes them ideal for use in remote or resource-limited settings, where access to laboratory-based diagnostic tests may be limited.
The growing focus on personalized medicine is also creating new opportunities in the market. Personalized medicine involves tailoring treatments to individual patients based on their unique genetic makeup, lifestyle, and other factors. The development of new diagnostic tests that can identify specific genetic markers associated with hepatitis E infection is paving the way for more personalized treatment options.

Market Dynamics
The global hepatitis E diagnostic tests market is highly dynamic, with several factors influencing its growth and development. The rising prevalence of hepatitis E infection worldwide, coupled with the increasing awareness about the disease and the need for early diagnosis and treatment, is driving the demand for hepatitis E diagnostic tests.
Advancements in diagnostic technologies, such as real-time PCR and NGS, are providing healthcare professionals with more accurate and reliable tools for diagnosing hepatitis E infection. However, the high cost of diagnostic tests and the limited availability of diagnostic tests in some regions are hindering the growth of the market.
Regional Analysis
North America is the largest market for hepatitis E diagnostic tests, followed by Europe and Asia Pacific. The high prevalence of hepatitis E in North America, coupled with the presence of several key players in the region, is driving the growth of the market.
In Europe, the increasing awareness about hepatitis E and the need for early diagnosis and treatment are driving the demand for diagnostic tests. The growing focus on personalized medicine is also creating new opportunities in the market.
Asia Pacific is expected to witness the highest growth rate during the forecast period, due to the rising prevalence of hepatitis E in the region. The increasing awareness about the disease and the need for early diagnosis and treatment are also driving the demand for hepatitis E diagnostic tests in the region.
Competitive Landscape
Leading companies in the Hepatitis E Diagnostic Tests Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Report Segmentation
The global hepatitis E diagnostic tests market can be segmented on the basis of test type, end user, and region. By test type, the market can be segmented into serology tests, nucleic acid amplification tests (NAAT), and enzyme-linked immunosorbent assays (ELISA).
By end user, the market can be segmented into hospitals, diagnostic laboratories, and research institutes. By region, the market can be segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
Category-wise Insights
Serology tests are the most commonly used diagnostic tests for hepatitis E. These tests detect antibodies produced by the body in response to the hepatitis E virus. Serology tests are relatively inexpensive and easy to perform, but they may not be accurate in certain situations.
NAAT, such as real-time PCR, can detect the presence of the hepatitis E virus directly in blood or other bodily fluids. These tests are more accurate than serology tests, but they are also more expensive and require specialized equipment and trained personnel.
ELISA is a type of serology test that uses enzymes to detect antibodies produced by the body in response to the hepatitis E virus. ELISA tests are highly sensitive and specific, but they can also produce false-positive results in patients with autoimmune diseases.
Key Benefits for Industry Participants and Stakeholders
The global hepatitis E diagnostic tests market offers several key benefits for industry participants and stakeholders. These include:
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Some of the key trends in the global hepatitis E diagnostic tests market include:
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the global hepatitis E diagnostic tests market. The outbreak of Covid-19 has led to a disruption in the supply chain and a reduction in the availability of diagnostic tests. Many companies operating in the market have also been forced to temporarily halt production or reduce production capacity due to the pandemic.
However, the pandemic has also created new opportunities in the market. The rising demand for diagnostic tests for Covid-19 has led to the development of new diagnostic technologies and an increased focus on point-of-care testing. This has paved the way for the development of new and improved diagnostic tests for other infectious diseases, including hepatitis E.
Key Industry Developments
Some of the key industry developments in the global hepatitis E diagnostic tests market include:
Analyst Suggestions
According to industry analysts, the global hepatitis E diagnostic tests market is expected to witness significant growth in the coming years, driven by the rising prevalence of hepatitis E infection worldwide and advancements in diagnostic technologies.
To stay competitive in the market, companies operating in the market are advised to focus on product development and innovation, and to invest in research and development to create new and improved diagnostic tests. Companies should also focus on expanding their product portfolios and entering into strategic partnerships and collaborations to gain a competitive edge in the market.
Future Outlook
The global hepatitis E diagnostic tests market is expected to witness significant growth in the coming years, driven by the rising prevalence of hepatitis E infection worldwide and advancements in diagnostic technologies. The increasing demand for point-of-care (POC) diagnostic tests and personalized medicine is also creating new opportunities in the market.
However, the high cost of diagnostic tests and the limited availability of diagnostic tests in some regions are major challenges facing the market. Companies operating in the market will need to focus on product development and innovation, and on expanding their product portfolios, to stay competitive in the market and to capitalize on the growing demand for hepatitis E diagnostic tests.
Conclusion
The global hepatitis E diagnostic tests market is expected to witness significant growth in the coming years, driven by the rising prevalence of hepatitis E infection worldwide and advancements in diagnostic technologies. The market is highly competitive, with several key players competing for market share. Serology tests, NAAT, and ELISA are the most commonly used diagnostic tests for hepatitis E.
North America is the largest market for hepatitis E diagnostic tests, followed by Europe and Asia Pacific. The market is expected to witness the highest growth rate in Asia Pacific during the forecast period, due to the rising prevalence of hepatitis E in the region.
What are Hepatitis E diagnostic tests?
Hepatitis E diagnostic tests are medical assessments used to detect the presence of the Hepatitis E virus in the body. These tests can identify viral RNA or antibodies, helping in the diagnosis and management of Hepatitis E infections.
What companies are leading the Hepatitis E diagnostic tests market?
Leading companies in the Hepatitis E diagnostic tests market include Abbott Laboratories, Roche Diagnostics, and Bio-Rad Laboratories, among others.
What are the key drivers of the Hepatitis E diagnostic tests market?
Key drivers of the Hepatitis E diagnostic tests market include the rising incidence of Hepatitis E infections, increased awareness about the disease, and advancements in diagnostic technologies that enhance test accuracy and speed.
What challenges does the Hepatitis E diagnostic tests market face?
The Hepatitis E diagnostic tests market faces challenges such as limited access to testing in low-resource settings, variability in test sensitivity and specificity, and the need for regulatory approvals for new diagnostic methods.
What opportunities exist in the Hepatitis E diagnostic tests market?
Opportunities in the Hepatitis E diagnostic tests market include the development of rapid and point-of-care testing solutions, expansion into emerging markets, and increased investment in research and development for innovative diagnostic technologies.
What trends are shaping the Hepatitis E diagnostic tests market?
Trends shaping the Hepatitis E diagnostic tests market include the integration of molecular diagnostics, the use of digital health technologies for remote testing, and a growing emphasis on personalized medicine approaches in infectious disease management.
Hepatitis E Diagnostic Tests Market
| Segmentation | Details |
|---|---|
| Test Type | Antibody Tests, Molecular Tests |
| End User | Hospitals, Diagnostic Laboratories, Research Centers, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Hepatitis E Diagnostic Tests Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at