444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The global plasma protease C1 inhibitor treatment market is a critical segment within the biopharmaceutical industry, focused on addressing hereditary angioedema (HAE) and other rare genetic disorders. This market encompasses the development, manufacturing, and distribution of C1 inhibitor therapies that play a crucial role in managing and preventing angioedema attacks. The market’s significance lies in providing life-saving treatments to individuals affected by these rare conditions, enhancing their quality of life and reducing the frequency and severity of debilitating attacks.
Meaning
Plasma protease C1 inhibitor treatment refers to a specific class of biopharmaceuticals designed to address hereditary angioedema (HAE) and related disorders. These therapies primarily target a deficiency or dysfunction in C1 inhibitor proteins, which play a vital role in regulating the body’s immune response. By replenishing or augmenting C1 inhibitor levels, these treatments aim to reduce the frequency and severity of angioedema attacks, which are characterized by sudden, severe swelling in various parts of the body.
These treatments are typically derived from human plasma or produced through recombinant DNA technology. They are administered intravenously and work by inhibiting the activity of enzymes responsible for triggering inappropriate immune responses. This intervention helps to maintain normal vascular permeability and prevent the uncontrolled release of inflammatory mediators, thereby mitigating the onset of angioedema symptoms.
Executive Summary
The global plasma protease C1 inhibitor treatment market has witnessed significant growth in recent years, driven by advancements in biopharmaceutical research and the increasing recognition of rare genetic disorders. This executive summary provides a concise overview of the market, highlighting key trends, treatment options, and future prospects.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
The plasma protease C1 inhibitor treatment market is characterized by several key insights that shape its current landscape and future growth potential.
Market Drivers
Several factors are driving the growth of the global plasma protease C1 inhibitor treatment market:
Market Restraints
Despite its growth, the global plasma protease C1 inhibitor treatment market faces certain challenges and restraints:
Market Opportunities
The global plasma protease C1 inhibitor treatment market offers several opportunities for growth and development:
Market Dynamics
The global plasma protease C1 inhibitor treatment market is characterized by dynamic factors that shape its growth and evolution:
Regional Analysis
The plasma protease C1 inhibitor treatment market exhibits regional variations in terms of demand, access to treatment, and market dynamics.
North America: North America, particularly the United States, holds a significant share of the market, driven by advanced healthcare infrastructure, research initiatives, and advocacy efforts.
Europe: Europe also boasts a well-established market for C1 inhibitor treatments, with a focus on access to advanced therapies and a strong patient advocacy presence.
Asia-Pacific: The Asia-Pacific region, led by countries like Japan, is experiencing growth in the market, driven by increasing awareness and improving access to treatments.
Middle East and Africa: The Middle East and Africa are gradually gaining traction in the market, with improving healthcare infrastructure and rising awareness of rare genetic disorders.
Competitive Landscape
Leading Companies in the Global Plasma Protease C1-inhibitor Treatment Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
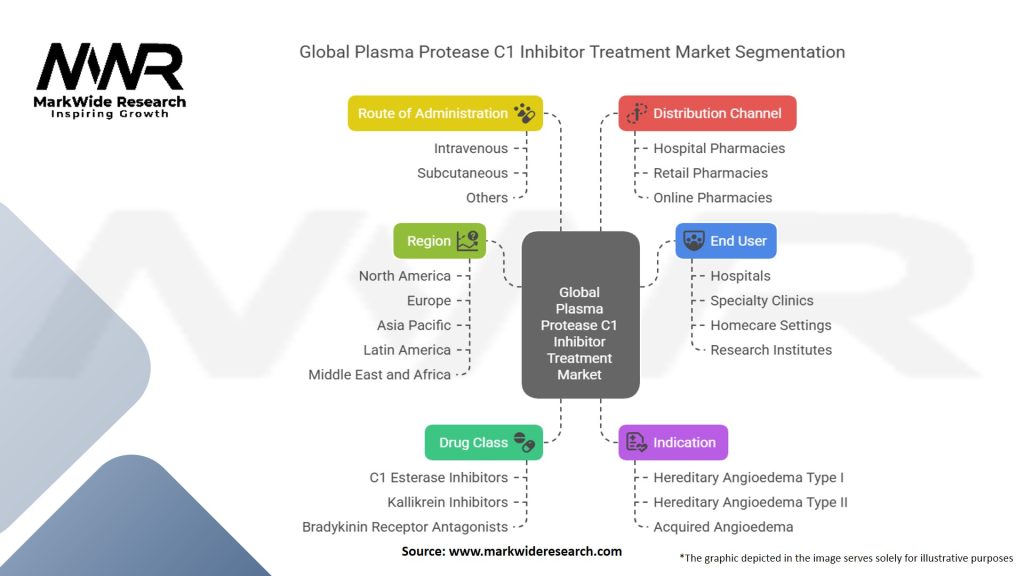
Segmentation
The global plasma protease C1 inhibitor treatment market can be segmented based on various factors to better understand its diverse offerings and application areas.
By Type of Treatment:
By Indication:
By Mode of Administration:
By Distribution Channel:
Segmentation allows market players to target specific treatment types, indications, and distribution channels effectively, considering the diverse needs of patients and healthcare providers.
Category-wise Insights
Each category within the plasma protease C1 inhibitor treatment market offers unique insights and considerations:
Plasma-Derived C1 Inhibitor: Plasma-derived C1 inhibitor therapies have a long history of use and provide effective management of hereditary angioedema (HAE). Ensuring a safe and stable supply of plasma for manufacturing is a critical consideration.
Recombinant C1 Inhibitor: Recombinant C1 inhibitor treatments offer promising alternatives with potential benefits such as reduced risk of infection transmission and improved accessibility for patients. Ongoing research and development in this category are essential for expanding treatment options.
Hereditary Angioedema (HAE): HAE represents a significant patient population, and advancements in HAE management, including better diagnostic tools and more convenient treatment options, are paramount.
Acquired Angioedema: Addressing acquired angioedema and other rare genetic disorders with C1 inhibitor therapies requires tailored approaches and further research into treatment effectiveness.
Mode of Administration: Considering the mode of administration is essential to enhance patient convenience and adherence to treatment plans. Subcutaneous administration, for example, offers potential advantages in certain cases.
Distribution Channels: Collaborations with healthcare institutions, specialty pharmacies, and online pharmacies are essential to ensure efficient treatment access for patients.
Key Benefits for Industry Participants and Stakeholders
Industry participants and stakeholders in the plasma protease C1 inhibitor treatment market can expect several key benefits:
SWOT Analysis
A SWOT analysis provides a comprehensive view of the plasma protease C1 inhibitor treatment market’s strengths, weaknesses, opportunities, and threats.
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
The plasma protease C1 inhibitor treatment market is witnessing several key trends that are shaping its trajectory:
Covid-19 Impact
The COVID-19 pandemic had a significant impact on the plasma protease C1 inhibitor treatment market. While the pandemic primarily focused attention on viral infections, healthcare systems faced challenges in providing care and treatment for patients with rare genetic disorders like hereditary angioedema (HAE).
The pandemic disrupted healthcare services, including routine diagnostic procedures, treatment administration, and patient follow-up. Patients with HAE faced difficulties in accessing treatment centers and managing their condition, which exacerbated the risk of angioedema attacks.
On the positive side, the pandemic underscored the importance of telehealth and remote monitoring, which allowed some patients to continue receiving care and treatment guidance. Telehealth also facilitated healthcare provider consultations and patient education.
The market for plasma protease C1 inhibitor treatment experienced some disruptions in the supply chain, affecting the availability of therapies. However, the pandemic also highlighted the resilience and adaptability of the biopharmaceutical industry, with manufacturers and healthcare providers working to ensure the continued availability of life-saving treatments.
As healthcare systems recover from the impact of the pandemic, there is renewed focus on improving access to C1 inhibitor therapies, enhancing patient education, and furthering research into rare genetic disorders.
Key Industry Developments
Several key industry developments have shaped the plasma protease C1 inhibitor treatment market in recent years:
Analyst Suggestions
Analysts offer several suggestions for industry participants and stakeholders in the plasma protease C1 inhibitor treatment market:
Future Outlook
The future of the plasma protease C1 inhibitor treatment market is promising, with several trends and factors shaping its trajectory:
Conclusion
In conclusion, the global plasma protease C1 inhibitor treatment market plays a crucial role in addressing rare genetic disorders, particularly hereditary angioedema (HAE), by providing life-saving therapies that improve the quality of life for affected individuals. The market has witnessed significant growth, driven by advancements in biopharmaceutical research, increased disease awareness, and regulatory support for rare disease treatments.
While the market faces challenges such as high treatment costs and the complexity of intravenous administration, it continues to evolve with innovations in treatment options, personalized medicine approaches, and expanded indications. Patient-centric care and global market expansion efforts are contributing to improved disease management and access to therapies.
As the market looks to the future, continued investment in research and development, collaboration among industry stakeholders, and engagement with regulatory authorities will be essential to advance treatment options and ensure the availability of life-saving C1 inhibitor therapies for individuals with rare genetic disorders. The industry’s commitment to innovation and patient well-being holds the promise of a brighter future for those affected by these conditions.
What is Global Plasma Protease C1 inhibitor Treatment?
Global Plasma Protease C1 inhibitor Treatment refers to therapies aimed at managing conditions related to the deficiency of the C1 inhibitor protein, which is crucial for regulating the complement and contact systems in the blood. These treatments are primarily used for conditions such as hereditary angioedema and other related disorders.
What are the key companies in the Global Plasma Protease C1 inhibitor Treatment Market?
Key companies in the Global Plasma Protease C1 inhibitor Treatment Market include CSL Behring, Shire (now part of Takeda), and BioCryst Pharmaceuticals, among others.
What are the growth factors driving the Global Plasma Protease C1 inhibitor Treatment Market?
The growth of the Global Plasma Protease C1 inhibitor Treatment Market is driven by the increasing prevalence of hereditary angioedema, advancements in treatment options, and rising awareness about the condition among healthcare professionals and patients.
What challenges does the Global Plasma Protease C1 inhibitor Treatment Market face?
The Global Plasma Protease C1 inhibitor Treatment Market faces challenges such as high treatment costs, limited patient access in certain regions, and the need for ongoing research to improve treatment efficacy and safety.
What opportunities exist in the Global Plasma Protease C1 inhibitor Treatment Market?
Opportunities in the Global Plasma Protease C1 inhibitor Treatment Market include the development of novel therapies, potential for combination treatments, and expanding market access in emerging economies.
What trends are shaping the Global Plasma Protease C1 inhibitor Treatment Market?
Trends shaping the Global Plasma Protease C1 inhibitor Treatment Market include the increasing focus on personalized medicine, advancements in biotechnology, and the growing use of telemedicine for patient management.
Global Plasma Protease C1 Inhibitor Treatment Market:
| Segmentation Details | Description |
|---|---|
| By Drug Class | C1 Esterase Inhibitors (Plasma-derived, Recombinant), Kallikrein Inhibitors, Bradykinin Receptor Antagonists |
| By Indication | Hereditary Angioedema (HAE) Type I, Hereditary Angioedema (HAE) Type II, Acquired Angioedema (AAE) |
| By Route of Administration | Intravenous, Subcutaneous, Others |
| By End User | Hospitals, Specialty Clinics, Homecare Settings, Research Institutes |
| By Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies |
| By Region | North America, Europe, Asia Pacific, Latin America, Middle East and Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Global Plasma Protease C1-inhibitor Treatment Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at