444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Wrist Fixator Procedure Kit market is witnessing substantial growth due to the rising incidence of wrist fractures, ligament injuries, and degenerative joint diseases. Wrist fixator procedure kits play a pivotal role in stabilizing and aiding the healing process of wrist fractures and other related conditions. These kits contain essential instruments and implants required for effective wrist fixation surgeries. The increasing adoption of minimally invasive surgical techniques and advancements in medical technology are further propelling the market’s expansion.
Meaning
A Wrist Fixator Procedure Kit refers to a set of medical devices and instruments designed to stabilize and support the wrist joint during surgical procedures. These kits are widely used by orthopedic surgeons to treat wrist fractures, dislocations, and other related injuries. They offer surgeons the necessary tools for precise and efficient wrist fixation, leading to improved patient outcomes and faster recovery.
Executive Summary
The Wrist Fixator Procedure Kit market is experiencing robust growth, driven by a growing geriatric population and a rise in sports-related injuries. This report provides a comprehensive analysis of the market, focusing on key market insights, drivers, restraints, opportunities, and trends. Additionally, the report assesses the impact of the COVID-19 pandemic on the market and presents a future outlook and analyst suggestions for industry participants and stakeholders.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Wrist Fixator Procedure Kit market is highly dynamic and influenced by several factors. The rising prevalence of wrist injuries and degenerative joint diseases is fueling market growth. Moreover, advancements in medical technology are enabling the development of more sophisticated and user-friendly procedure kits. However, challenges such as the high cost of procedure kits and limited reimbursement policies are impeding market growth. Despite these challenges, emerging markets present lucrative opportunities for market players to expand their presence and gain a competitive edge.
Regional Analysis
The market for Wrist Fixator Procedure Kits is geographically segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. North America holds a significant share in the market due to the region’s well-established healthcare infrastructure and high adoption of advanced medical technologies. Europe follows closely, driven by a large patient pool and increasing investments in orthopedic surgeries. The Asia Pacific region is anticipated to witness rapid growth due to the rising prevalence of wrist injuries and increasing healthcare expenditure in countries like India and China.
Competitive Landscape
Leading Companies in Wrist Fixator Procedure Kit Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
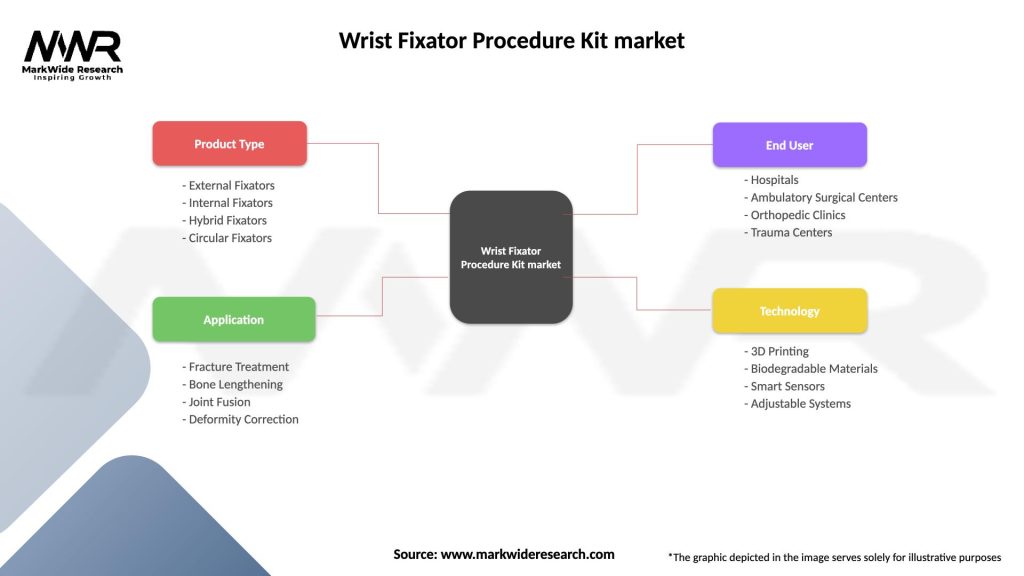
The market for Wrist Fixator Procedure Kits can be segmented based on product type, end-user, and region. By product type, the segments include external fixator procedure kits and internal fixator procedure kits. Based on end-users, the market is segmented into hospitals, ambulatory surgical centers, and orthopedic clinics.
Category-wise Insights
External Fixator Procedure Kits are witnessing significant demand due to their versatility and ease of application. They are preferred for open fractures and complex wrist injuries, providing stability and support during the healing process. On the other hand, Internal Fixator Procedure Kits are gaining traction due to advancements in implant materials and design, leading to reduced post-operative complications.
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a mixed impact on the Wrist Fixator Procedure Kit market. While elective surgeries experienced a downturn due to healthcare priorities shifting to COVID-19 treatment, the demand for trauma-related procedures, including wrist fixation surgeries, remained steady. As healthcare systems recover from the pandemic, the market is expected to witness renewed growth.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Wrist Fixator Procedure Kit market is expected to grow steadily in the coming years, driven by the rising incidence of wrist injuries and advancements in medical technology. Market players should focus on R&D to develop more efficient and cost-effective procedure kits to meet the evolving needs of healthcare professionals and patients. Additionally, expanding their presence in emerging markets will prove beneficial in securing a competitive advantage.
Conclusion
The Wrist Fixator Procedure Kit market presents immense opportunities for growth, fueled by an increasing number of wrist injuries, advancements in medical technology, and the rise in minimally invasive surgeries. Although the market faces challenges such as high costs and limited reimbursement policies, strategic efforts to innovate and expand into emerging markets can pave the way for success. As the healthcare industry continues to evolve, wrist fixator procedure kits will play a pivotal role in improving patient outcomes and enhancing overall quality of life.
What is Wrist Fixator Procedure Kit?
A Wrist Fixator Procedure Kit is a collection of medical instruments and devices specifically designed for the stabilization and fixation of wrist fractures and injuries during surgical procedures. These kits typically include various types of fixators, screws, and tools necessary for effective treatment.
What are the key companies in the Wrist Fixator Procedure Kit market?
Key companies in the Wrist Fixator Procedure Kit market include Stryker Corporation, Zimmer Biomet, and DePuy Synthes, among others. These companies are known for their innovative products and solutions in orthopedic surgery.
What are the growth factors driving the Wrist Fixator Procedure Kit market?
The growth of the Wrist Fixator Procedure Kit market is driven by the increasing incidence of wrist fractures, advancements in surgical techniques, and the rising demand for minimally invasive procedures. Additionally, the aging population contributes to a higher prevalence of orthopedic injuries.
What challenges does the Wrist Fixator Procedure Kit market face?
The Wrist Fixator Procedure Kit market faces challenges such as the high cost of advanced surgical kits and the need for skilled professionals to perform complex procedures. Furthermore, regulatory hurdles can delay the introduction of new products.
What opportunities exist in the Wrist Fixator Procedure Kit market?
Opportunities in the Wrist Fixator Procedure Kit market include the development of innovative fixation technologies and the expansion of product offerings to cater to emerging markets. Additionally, increasing awareness about orthopedic health can drive demand for these kits.
What trends are shaping the Wrist Fixator Procedure Kit market?
Trends in the Wrist Fixator Procedure Kit market include the integration of smart technology in surgical instruments and the growing preference for personalized medicine. There is also a shift towards the use of biodegradable materials in the manufacturing of surgical kits.
Wrist Fixator Procedure Kit market
| Segmentation Details | Description |
|---|---|
| Product Type | External Fixators, Internal Fixators, Hybrid Fixators, Circular Fixators |
| Application | Fracture Treatment, Bone Lengthening, Joint Fusion, Deformity Correction |
| End User | Hospitals, Ambulatory Surgical Centers, Orthopedic Clinics, Trauma Centers |
| Technology | 3D Printing, Biodegradable Materials, Smart Sensors, Adjustable Systems |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Wrist Fixator Procedure Kit Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at