444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Pharmaceuticals Serialization market is a rapidly growing sector within the pharmaceutical industry. Serialization refers to the process of assigning a unique identifier to individual pharmaceutical products, such as a barcode or a QR code, to ensure traceability and prevent counterfeiting. This market is driven by the increasing need for pharmaceutical companies to comply with regulatory requirements and improve patient safety.
Meaning
Pharmaceuticals serialization involves the implementation of unique identification codes on pharmaceutical products and their packaging. These codes enable the tracking and tracing of products throughout the supply chain, from manufacturing to distribution and even to the end consumer. Serialization helps to prevent the entry of counterfeit drugs into the market, safeguard patient health, and enhance overall pharmaceutical supply chain efficiency.
Executive Summary
The Pharmaceuticals Serialization market is witnessing significant growth due to the rising demand for product traceability and patient safety. The implementation of serialization measures has become crucial for pharmaceutical companies to comply with stringent regulations and combat the menace of counterfeit drugs. This market provides opportunities for technology providers, software developers, and packaging companies to offer innovative solutions and services.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
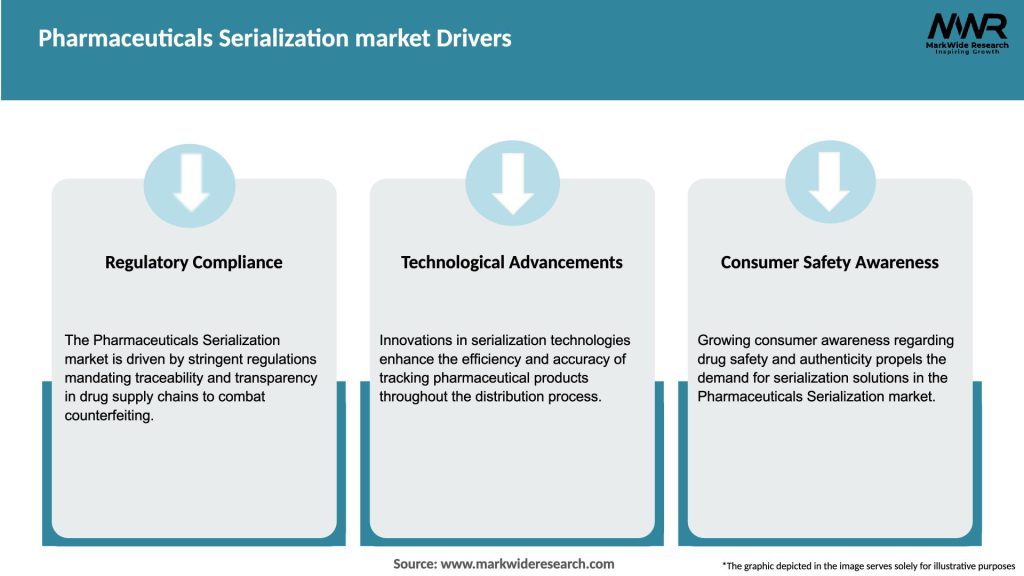
Market Drivers
Market Restraints
Market Opportunities

Market Dynamics
The Pharmaceuticals Serialization market is driven by regulatory compliance, the need to combat counterfeit drugs, and concerns about patient safety. However, the market faces challenges related to implementation costs, lack of global harmonization, resistance to change, and data management complexities. Despite these challenges, there are significant opportunities in emerging markets, the integration of emerging technologies, customized solutions, and collaboration among industry stakeholders.
Regional Analysis
The Pharmaceuticals Serialization market is geographically segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America and Europe are leading regions due to stringent regulatory requirements and a mature pharmaceutical industry. The Asia Pacific region is expected to witness significant growth due to the increasing adoption of serialization measures in countries like China and India. Latin America and the Middle East and Africa are also emerging markets, driven by rising awareness of the need for serialization.
Competitive Landscape
Leading Companies in Pharmaceuticals Serialization Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Pharmaceuticals Serialization market can be segmented based on technology, application, and end-user.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the Pharmaceuticals Serialization market. The pandemic highlighted the vulnerabilities in the global pharmaceutical supply chain and increased the urgency to strengthen traceability and supply chain resilience. The need for serialization to ensure the authenticity and safety of pharmaceutical products has been further emphasized. Pharmaceutical companies are investing in serialization solutions to mitigate the risks posed by counterfeit drugs and enhance their supply chain capabilities.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Pharmaceuticals Serialization market is expected to witness substantial growth in the coming years. Increasing regulatory requirements, rising concerns about patient safety, and the need to combat counterfeit drugs will continue to drive the demand for serialization solutions. The integration of emerging technologies, customization of solutions, and collaboration among stakeholders will shape the future of the market. Emerging markets, especially in Asia Pacific, offer significant growth opportunities. As serialization becomes an integral part of the pharmaceutical industry, companies that invest in innovative and scalable solutions will gain a competitive edge.
Conclusion
The Pharmaceuticals Serialization market is experiencing significant growth driven by regulatory compliance, the need to combat counterfeit drugs, and concerns about patient safety. Despite challenges related to implementation costs, lack of harmonization, and data management complexities, the market presents opportunities in emerging markets, integration of emerging technologies, customized solutions, and collaboration among industry stakeholders. Continuous advancements in serialization technologies, evolving regulations, and increased awareness will shape the future of the market. Pharmaceutical companies need to adapt to these trends, invest in scalable solutions, and collaborate with supply chain partners to ensure efficient serialization implementation and enhance supply chain visibility.
What is Pharmaceuticals Serialization?
Pharmaceuticals Serialization refers to the process of assigning a unique identifier to each saleable unit of a pharmaceutical product. This practice enhances traceability and helps in combating counterfeit drugs in the supply chain.
What are the key players in the Pharmaceuticals Serialization market?
Key players in the Pharmaceuticals Serialization market include companies like Siemens, Optel Group, and Systech International, which provide serialization solutions and technologies for the pharmaceutical industry, among others.
What are the main drivers of the Pharmaceuticals Serialization market?
The main drivers of the Pharmaceuticals Serialization market include the increasing need for drug traceability, regulatory requirements for serialization, and the rising incidence of counterfeit drugs. These factors are pushing pharmaceutical companies to adopt serialization technologies.
What challenges does the Pharmaceuticals Serialization market face?
The Pharmaceuticals Serialization market faces challenges such as the high costs of implementation, the complexity of integrating serialization systems with existing processes, and the need for compliance with varying regulations across different regions.
What opportunities exist in the Pharmaceuticals Serialization market?
Opportunities in the Pharmaceuticals Serialization market include advancements in blockchain technology for enhanced security, the growing demand for supply chain transparency, and the potential for serialization solutions to be applied in emerging markets.
What trends are shaping the Pharmaceuticals Serialization market?
Trends shaping the Pharmaceuticals Serialization market include the increasing adoption of cloud-based serialization solutions, the integration of IoT for real-time tracking, and the focus on sustainability in packaging and supply chain processes.
Pharmaceuticals Serialization market
| Segmentation Details | Description |
|---|---|
| Product Type | Serialization Software, Serialization Hardware, Labeling Solutions, Track & Trace Systems |
| End User | Pharmaceutical Manufacturers, Contract Packaging Organizations, Distributors, Retail Pharmacies |
| Technology | 2D Barcodes, RFID, Digital Watermarking, Blockchain |
| Application | Supply Chain Management, Regulatory Compliance, Inventory Management, Quality Control |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Pharmaceuticals Serialization Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at