444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
Lateral Flow Immunoassay (LFIA) based rapid tests have emerged as a game-changer in the field of diagnostic testing. These tests provide quick and reliable results, making them highly valuable in various industries, including healthcare, food safety, veterinary diagnostics, and environmental testing. The LFIA market has witnessed significant growth in recent years, driven by the increasing demand for point-of-care testing, rising prevalence of infectious diseases, and advancements in technology.
Meaning
Lateral Flow Immunoassay (LFIA) based rapid tests are diagnostic tools that provide quick and reliable results for various diseases and conditions. These tests utilize the principles of immunochromatography, enabling the detection of specific analytes such as antibodies or antigens in a sample. LFIA tests are widely used in healthcare settings, point-of-care testing, and home diagnostics due to their simplicity, cost-effectiveness, and rapid turnaround time.
Executive Summary
The global market for LFIA-based rapid tests has witnessed substantial growth in recent years. The increasing demand for point-of-care diagnostics, the need for quick and accurate results, and the rising prevalence of infectious diseases have driven the market’s expansion. This report provides key insights into the LFIA-based rapid test market, including market drivers, restraints, opportunities, regional analysis, competitive landscape, segmentation, key trends, COVID-19 impact, industry developments, analyst suggestions, future outlook, and a comprehensive conclusion.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The LFIA-based rapid test market is characterized by intense competition among key players, technological advancements, and strategic collaborations. The market is driven by the increasing demand for rapid and accurate diagnostics, especially in point-of-care settings. However, challenges such as maintaining test sensitivity and specificity, regulatory complexities, and competition from alternative diagnostic methods pose obstacles to market growth. Despite these challenges, opportunities lie in the expansion of LFIA-based tests for emerging diseases, home diagnostics, and collaborations with healthcare providers.
Regional Analysis
The LFIA-based rapid test market is segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America dominates the market due to the presence of key market players, advanced healthcare infrastructure, and high adoption of novel technologies. Europe follows closely, driven by increasing awareness, government initiatives, and advancements in healthcare. The Asia Pacific region is expected to witness significant growth, driven by the rising prevalence of infectious diseases, expanding healthcare infrastructure, and increasing focus on point-of-care testing.
Competitive Landscape
Leading Companies in the Lateral Flow Immunoassay (LFIA) Based Rapid Test Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

Segmentation
The LFIA-based rapid test market can be segmented based on application, end-user, and region. By application, the market can be divided into infectious diseases, pregnancy and fertility, drug abuse testing, cardiac markers, and others. By end-user, the market includes hospitals and clinics, home diagnostics, diagnostic centers, and others. Geographically, the market can be categorized into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
COVID-19 Impact
The COVID-19 pandemic has significantly impacted the LFIA-based rapid test market. The urgent need for mass testing, contact tracing, and surveillance has led to a surge in demand for rapid antigen tests. LFIA-based rapid tests have played a crucial role in diagnosing and monitoring COVID-19 cases, both in healthcare settings and for self-testing at home. The pandemic has accelerated research and development efforts in the LFIA field, leading to advancements and improvements in test sensitivity, specificity, and ease of use.
Key Industry Developments
Analyst Suggestions
Future Outlook
The LFIA-based rapid test market is expected to witness substantial growth in the coming years. Factors such as increasing demand for rapid diagnostics, advancements in technology, and the rising prevalence of infectious diseases will drive market expansion. However, challenges related to test limitations and regulatory complexities need to be addressed. Opportunities lie in the expansion of LFIA tests for emerging diseases, home diagnostics, and collaborations with healthcare providers. The market is likely to witness further consolidation, technological advancements, and strategic partnerships.
Conclusion
The LFIA-based rapid test market is a rapidly growing sector in the diagnostic industry. The simplicity, cost-effectiveness, and quick turnaround time of LFIA tests have positioned them as essential tools for point-of-care diagnostics and home testing. While the market faces challenges such as maintaining test sensitivity and specificity, regulatory complexities, and competition from alternative methods, opportunities exist in the expansion of LFIA tests for emerging diseases, home diagnostics, and collaborations with healthcare providers. With advancements in technology, the LFIA-based rapid test market is poised for significant growth, contributing to improved healthcare outcomes globally.
What is Lateral Flow Immunoassay (LFIA) Based Rapid Test?
Lateral Flow Immunoassay (LFIA) Based Rapid Test is a diagnostic tool that uses immunoassay technology to detect specific substances in a sample, typically providing results quickly and without the need for specialized equipment. These tests are commonly used in medical diagnostics, food safety, and environmental monitoring.
What are the key companies in the Lateral Flow Immunoassay (LFIA) Based Rapid Test market?
Key companies in the Lateral Flow Immunoassay (LFIA) Based Rapid Test market include Abbott Laboratories, Roche Diagnostics, and Quidel Corporation, among others.
What are the growth factors driving the Lateral Flow Immunoassay (LFIA) Based Rapid Test market?
The growth of the Lateral Flow Immunoassay (LFIA) Based Rapid Test market is driven by the increasing demand for rapid diagnostic tests, the rise in infectious diseases, and advancements in LFIA technology that enhance test accuracy and usability.
What challenges does the Lateral Flow Immunoassay (LFIA) Based Rapid Test market face?
Challenges in the Lateral Flow Immunoassay (LFIA) Based Rapid Test market include the need for regulatory approvals, potential issues with test sensitivity and specificity, and competition from other diagnostic methods.
What opportunities exist in the Lateral Flow Immunoassay (LFIA) Based Rapid Test market?
Opportunities in the Lateral Flow Immunoassay (LFIA) Based Rapid Test market include the development of tests for emerging diseases, expansion into point-of-care testing, and increasing investments in healthcare infrastructure.
What trends are shaping the Lateral Flow Immunoassay (LFIA) Based Rapid Test market?
Trends in the Lateral Flow Immunoassay (LFIA) Based Rapid Test market include the integration of digital technologies for result interpretation, the development of multiplex testing capabilities, and a growing focus on home-based testing solutions.
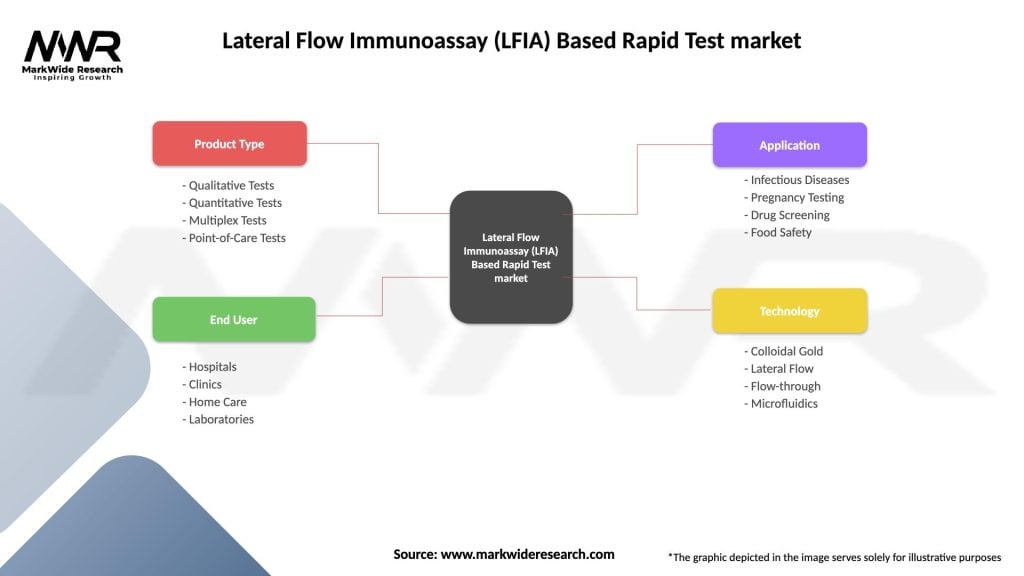
Lateral Flow Immunoassay (LFIA) Based Rapid Test market
| Segmentation Details | Description |
|---|---|
| Product Type | Qualitative Tests, Quantitative Tests, Multiplex Tests, Point-of-Care Tests |
| End User | Hospitals, Clinics, Home Care, Laboratories |
| Application | Infectious Diseases, Pregnancy Testing, Drug Screening, Food Safety |
| Technology | Colloidal Gold, Lateral Flow, Flow-through, Microfluidics |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Lateral Flow Immunoassay (LFIA) Based Rapid Test Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at