444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The global HPV (Human Papillomavirus) testing and Pap test market has witnessed significant growth in recent years. These tests play a crucial role in the early detection and prevention of cervical cancer, a leading cause of cancer-related deaths among women worldwide. HPV testing helps identify the presence of high-risk HPV strains, while Pap tests examine cervical cells for abnormalities. With the increasing awareness about the importance of early detection and advancements in diagnostic technologies, the HPV testing and Pap test market is expected to experience substantial growth in the forecast period.
Meaning
HPV testing and Pap tests are diagnostic procedures used to detect abnormalities in cervical cells and identify the presence of high-risk HPV strains. The human papillomavirus is a common sexually transmitted infection that can lead to cervical cancer. HPV testing involves analyzing a cervical sample for the presence of viral DNA or RNA. On the other hand, Pap tests involve collecting cells from the cervix and examining them under a microscope for signs of abnormality or precancerous changes. These tests are crucial in screening and early detection, allowing for timely intervention and reducing the risk of cervical cancer.
Executive Summary
The global HPV testing and Pap test market is witnessing steady growth, driven by the increasing awareness about cervical cancer prevention and early detection. These tests play a vital role in identifying high-risk HPV strains and detecting abnormal cervical cells, leading to timely interventions and improved patient outcomes. The market is characterized by advancements in diagnostic technologies, increasing investments in healthcare infrastructure, and government initiatives to promote cervical cancer screening. The market shows significant potential for growth in both developed and emerging economies. Despite the COVID-19 pandemic impacting healthcare systems worldwide, the HPV testing and Pap test market has remained resilient.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
The HPV testing and Pap test market is driven by several factors that contribute to its growth and expansion. Some key market drivers include:
Market Restraints
While the HPV testing and Pap test market exhibits promising growth potential, it is not without its challenges. Some of the key market restraints include:
Market Opportunities
The HPV testing and Pap test market present several opportunities for industry participants to capitalize on. These opportunities include:
Market Dynamics
The HPV testing and Pap test market is dynamic and influenced by various factors, including technological advancements, healthcare policies, and changing consumer behaviors. Understanding the market dynamics is crucial for industry participants to identify opportunities, mitigate risks, and stay competitive.
Regional Analysis
The global HPV testing and Pap test market exhibit regional variations in terms of market size, growth potential, and market dynamics. Understanding the regional landscape is essential for industry participants to identify key markets and tailor their strategies accordingly.
North America: North America dominates the HPV testing and Pap test market, attributed to the well-established healthcare infrastructure, high awareness levels, and strong government support for cervical cancer screening. The presence of major market players and advanced diagnostic technologies further contribute to market growth in this region.
Europe: Europe is another significant market for HPV testing and Pap tests. The region emphasizes preventive healthcare and has implemented organized screening programs in many countries. The adoption of evidence-based practices, quality assurance measures, and standardized guidelines drive the market growth in Europe.
Asia Pacific: The Asia Pacific region presents significant growth opportunities for the HPV testing and Pap test market. The region has a large population, particularly in countries like India and China, where the burden of cervical cancer is high. Increasing healthcare expenditure, improving healthcare infrastructure, and rising awareness of preventive healthcare contribute to market growth in Asia Pacific.
Latin America: Latin America has witnessed improvements in cervical cancer screening programs and the adoption of HPV testing and Pap tests. Efforts by governments and healthcare organizations to raise awareness, expand access to screening, and implement vaccination programs drive the market growth in this region.
Middle East and Africa: The Middle East and Africa region present untapped potential for the HPV testing and Pap test market. The prevalence of cervical cancer is relatively high in certain countries in this region. Initiatives to improve healthcare infrastructure, raise awareness, and strengthen screening programs can drive the market growth in the Middle East and Africa.
Competitive Landscape
Leading Companies in Global HPV Testing and Pap Test Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
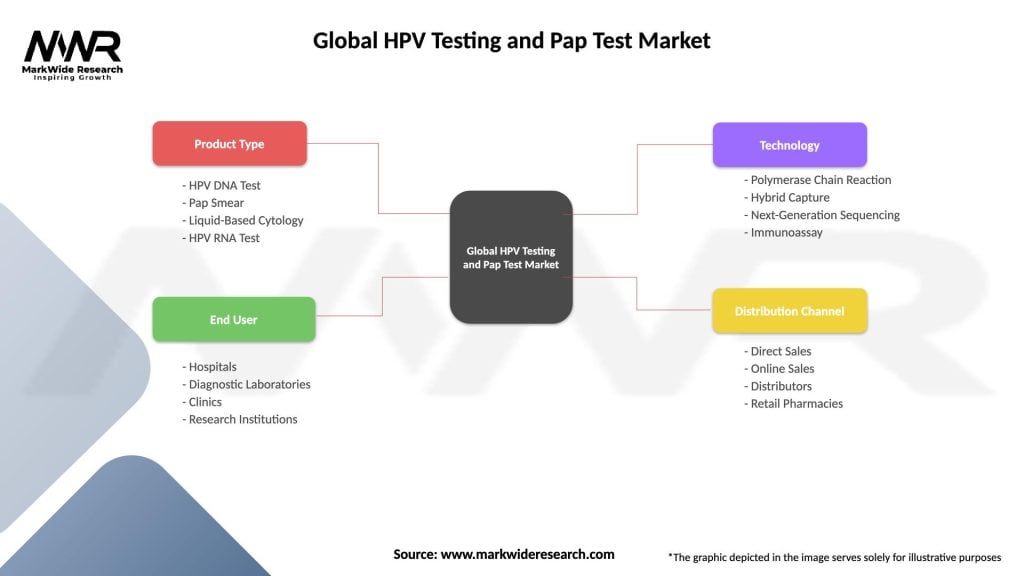
The HPV testing and Pap test market can be segmented based on various factors such as test type, end-user, and geography.
Category-wise Insights
HPV Testing Segment: The HPV testing segment holds a significant share in the market. HPV testing is a crucial component of cervical cancer screening, allowing for the early detection of high-risk HPV strains. The adoption of HPV testing has increased due to its high sensitivity and specificity in identifying individuals at risk of developing cervical cancer.
Pap Test (Cervical Cytology) Segment: The Pap test segment remains a widely used screening method for cervical cancer. The Pap test involves the examination of cervical cells under a microscope to detect abnormalities or precancerous changes. It plays a critical role in identifying cellular abnormalities, leading to further evaluation and appropriate interventions.
Hospitals End-User Segment: Hospitals are the primary end-users of HPV testing and Pap tests, owing to their comprehensive healthcare services, advanced diagnostic facilities, and multidisciplinary approach to cancer management. Hospitals play a pivotal role in cervical cancer screening and the management of abnormal results.
Clinics and Diagnostic Centers End-User Segment: Clinics and diagnostic centers cater to the increasing demand for preventive healthcare services, including cervical cancer screening. These facilities offer convenience, accessibility, and specialized expertise in conducting HPV testing and Pap tests.
Research Institutes End-User Segment: Research institutes play a crucial role in advancing the field of cervical cancer screening and improving the diagnostic accuracy of HPV testing and Pap tests. These institutions conduct clinical trials, develop new screening technologies, and contribute to evidence-based guidelines.
Key Benefits for Industry Participants and Stakeholders
The HPV testing and Pap test market offer several benefits for industry participants and stakeholders:
SWOT Analysis
A SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis provides a comprehensive understanding of the HPV testing and Pap test market’s internal and external factors.
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
The HPV testing and Pap test market are influenced by several key trends:
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the HPV testing and Pap test market. While the pandemic disrupted healthcare services worldwide, including routine screenings, efforts were made to ensure continuity of cervical cancer screening programs.
During the pandemic, the focus on preventive healthcare and early detection remained crucial. Healthcare providers adopted measures such as telemedicine consultations, prioritization of high-risk cases, and implementation of safety protocols to ensure the delivery of HPV testing and Pap tests.
The pandemic also highlighted the importance of accessible and decentralized screening options. Self-sampling kits gained attention as a means to overcome barriers to screening, especially during lockdowns and restricted movement.
However, the impact of the pandemic on the market varied across regions depending on the severity of outbreaks, healthcare system capacities, and resource allocations. As the situation stabilizes and healthcare services resume, the HPV testing and Pap test market is expected to recover and continue its growth trajectory.
Key Industry Developments
The HPV testing and Pap test market have witnessed significant industry developments, including:
Analyst Suggestions
Based on the current market dynamics, analysts suggest the following strategies for industry participants:
Future Outlook
The future outlook for the global HPV testing and Pap test market remains positive. The market is expected to witness steady growth, driven by increasing awareness about cervical cancer prevention, advancements in diagnostic technologies, and expanding healthcare infrastructure.
Efforts to improve access to screening services in underserved regions and the integration of digital solutions will contribute to market growth. The adoption of point-of-care testing and the development of AI-based screening tools hold promise for enhancing screening coverage and diagnostic accuracy.
However, challenges such as limited accessibility in developing regions, pricing pressures, and social barriers need to be addressed. Industry participants must adapt to evolving regulatory requirements, reimbursement policies, and changing market dynamics to stay competitive.
Conclusion
The HPV testing and Pap test market play a vital role in cervical cancer screening and prevention. With ongoing advancements in technology, increased awareness, and collaborations between industry participants and healthcare organizations, the market is poised for continued growth and improved patient outcomes.
What is HPV Testing and Pap Test?
HPV Testing and Pap Test are medical procedures used to screen for cervical cancer and human papillomavirus (HPV) infections. These tests help in early detection and prevention of cervical cancer by identifying abnormal cells and the presence of HPV, which is linked to the disease.
What are the key players in the Global HPV Testing and Pap Test Market?
Key players in the Global HPV Testing and Pap Test Market include Roche Diagnostics, Hologic, and Qiagen, which are known for their innovative testing solutions and technologies. These companies focus on enhancing the accuracy and efficiency of HPV testing and Pap tests, among others.
What are the growth factors driving the Global HPV Testing and Pap Test Market?
The Global HPV Testing and Pap Test Market is driven by increasing awareness about cervical cancer screening, advancements in testing technologies, and rising healthcare expenditure. Additionally, government initiatives promoting regular screenings contribute to market growth.
What challenges does the Global HPV Testing and Pap Test Market face?
The Global HPV Testing and Pap Test Market faces challenges such as the high cost of advanced testing technologies and a lack of awareness in certain regions. Additionally, variations in healthcare infrastructure can hinder access to these essential tests.
What opportunities exist in the Global HPV Testing and Pap Test Market?
Opportunities in the Global HPV Testing and Pap Test Market include the development of more affordable testing options and the integration of digital health technologies. Furthermore, expanding access to testing in underserved areas presents significant growth potential.
What trends are shaping the Global HPV Testing and Pap Test Market?
Trends shaping the Global HPV Testing and Pap Test Market include the increasing adoption of self-collection methods for HPV testing and the use of molecular diagnostics. Additionally, there is a growing emphasis on personalized medicine and targeted therapies in cervical cancer prevention.
Global HPV Testing and Pap Test Market
| Segmentation Details | Description |
|---|---|
| Product Type | HPV DNA Test, Pap Smear, Liquid-Based Cytology, HPV RNA Test |
| End User | Hospitals, Diagnostic Laboratories, Clinics, Research Institutions |
| Technology | Polymerase Chain Reaction, Hybrid Capture, Next-Generation Sequencing, Immunoassay |
| Distribution Channel | Direct Sales, Online Sales, Distributors, Retail Pharmacies |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Global HPV Testing and Pap Test Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at