444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The 3D printing medical device software market has witnessed significant growth in recent years, driven by advancements in technology and the increasing adoption of 3D printing in the healthcare industry. This market encompasses software solutions that enable the design, modeling, and manufacturing of medical devices using 3D printing technology. The software plays a crucial role in transforming digital designs into physical objects, facilitating personalized and patient-specific medical device production.
Meaning
3D printing medical device software refers to the computer programs and tools used to create digital models, design medical devices, and control the 3D printing process. These software solutions enable healthcare professionals, researchers, and manufacturers to develop innovative medical devices with improved functionality, customization, and cost-effectiveness.
Executive Summary
The 3D printing medical device software market is experiencing substantial growth due to the increasing demand for personalized healthcare solutions and the need for efficient and accurate medical device production. The software provides healthcare professionals with the ability to customize medical devices according to individual patient needs, resulting in enhanced treatment outcomes and patient satisfaction. Additionally, the integration of 3D printing technology in the medical device manufacturing process improves productivity, reduces costs, and enables faster prototyping and production.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The 3D printing medical device software market is driven by a combination of technological advancements, increasing demand for personalized healthcare, and cost-effectiveness in manufacturing processes. However, the market also faces challenges related to regulatory compliance, intellectual property protection, and limited material options. These dynamics shape the competitive landscape and market opportunities for industry participants.
Regional Analysis
The market for 3D printing medical device software is experiencing significant growth globally, with North America, Europe, Asia Pacific, and the Rest of the World being key regions. North America dominates the market due to the presence of advanced healthcare infrastructure, substantial investments in research and development, and a supportive regulatory environment. Europe follows closely, driven by increasing adoption of 3D printing in healthcare and a strong focus on personalized medicine. The Asia Pacific region is witnessing rapid growth due to rising healthcare expenditure, growing awareness of 3D printing technology, and a large patient population.
Competitive Landscape
Leading Companies in the 3D Printing Medical Device Software Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
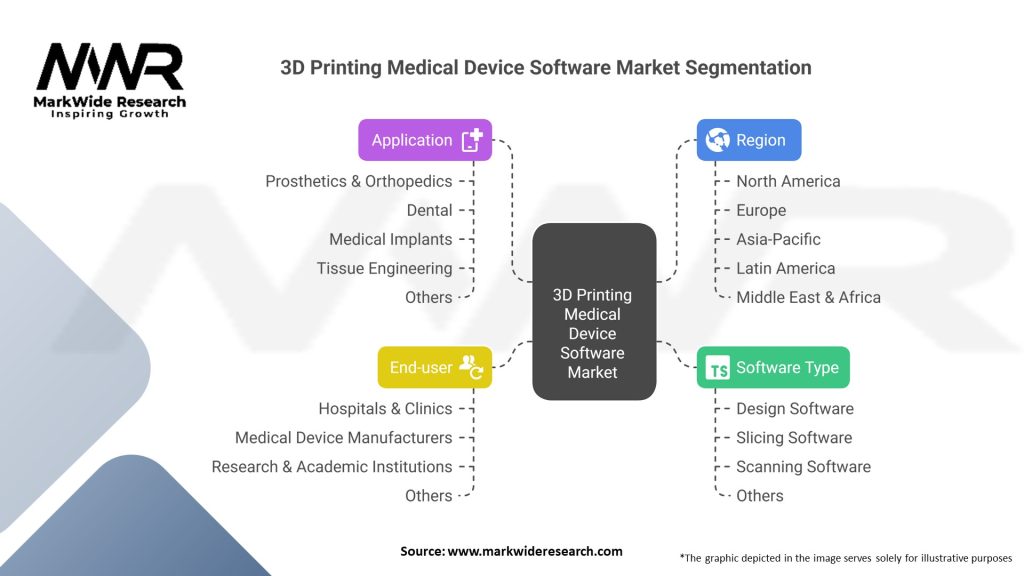
The 3D printing medical device software market can be segmented based on software type, end-user, and application. Software types include design software, modeling software, and control software. End-users comprise hospitals and surgical centers, dental clinics, research institutions, and medical device manufacturers. Applications include orthopedics, dental, prosthetics, implants, and surgical guides, among others. These segments allow for a comprehensive analysis of the market, understanding specific software requirements, and addressing the diverse needs of end-users.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has highlighted the importance of 3D printing medical device software in addressing healthcare challenges. During the pandemic, 3D printing technology played a crucial role in producing personal protective equipment (PPE), ventilator components, and other medical devices. The flexibility and rapid production capabilities of 3D printing software have proven invaluable in responding to the urgent need for medical supplies.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the 3D printing medical device software market looks promising. The demand for personalized healthcare solutions, advancements in 3D printing technology, and the increasing adoption of 3D printing in the healthcare industry will continue to drive market growth. As the regulatory landscape becomes more defined and standards for 3D printed medical devices are established, the market will experience further expansion.
The integration of artificial intelligence (AI) and machine learning (ML) technologies into 3D printing medical device software will unlock new possibilities for design optimization, automated workflows, and predictive analytics. This will lead to increased efficiency, accuracy, and productivity in the manufacturing process.
Moreover, advancements in materials for 3D printing, including biocompatible and sterilizable options, will broaden the range of medical devices that can be produced using this technology. The development of high-performance materials will further enhance the functionality and durability of 3D printed medical devices, opening doors to new applications and improved patient care.
The market will also witness increased competition as more players enter the industry. Established software providers will focus on continuous innovation and expanding their product offerings to maintain their market share. At the same time, startups and smaller companies will bring fresh ideas and niche solutions to the market, fostering healthy competition and pushing the boundaries of what can be achieved with 3D printing medical device software.
Conclusion
The 3D printing medical device software market is poised for significant growth as the demand for personalized healthcare solutions and cost-effective manufacturing processes continues to rise. The ability to create customized, patient-specific medical devices through 3D printing software is revolutionizing the healthcare industry, leading to improved treatment outcomes and enhanced patient satisfaction.
While the market faces challenges related to regulations, intellectual property, and limited material options, opportunities abound. Collaboration with healthcare institutions, emerging applications in complex surgeries, and advancements in software capabilities present avenues for market expansion.
Industry participants should focus on regulatory compliance, invest in research and development, and foster collaborations to stay ahead in this dynamic and competitive market. By embracing these strategies, the 3D printing medical device software market can unlock its full potential and continue to revolutionize the healthcare industry for years to come.
What is 3D Printing Medical Device Software?
3D Printing Medical Device Software refers to specialized applications that facilitate the design, simulation, and production of medical devices using 3D printing technology. This software is essential for creating customized implants, prosthetics, and surgical tools tailored to individual patient needs.
What are the key players in the 3D Printing Medical Device Software market?
Key players in the 3D Printing Medical Device Software market include Stratasys, Materialise, and 3D Systems, which provide innovative solutions for medical applications. These companies focus on enhancing the capabilities of 3D printing in healthcare, among others.
What are the growth factors driving the 3D Printing Medical Device Software market?
The growth of the 3D Printing Medical Device Software market is driven by the increasing demand for personalized medical solutions, advancements in 3D printing technology, and the rising prevalence of chronic diseases requiring customized treatments. Additionally, the cost-effectiveness of 3D printing is attracting more healthcare providers.
What challenges does the 3D Printing Medical Device Software market face?
Challenges in the 3D Printing Medical Device Software market include regulatory hurdles, the need for standardization in software and materials, and concerns regarding the quality and safety of printed medical devices. These factors can hinder widespread adoption in clinical settings.
What opportunities exist in the 3D Printing Medical Device Software market?
Opportunities in the 3D Printing Medical Device Software market include the potential for innovations in bioprinting, the development of software for new applications such as tissue engineering, and collaborations between software developers and healthcare providers to enhance patient outcomes.
What trends are shaping the 3D Printing Medical Device Software market?
Trends in the 3D Printing Medical Device Software market include the integration of artificial intelligence for improved design processes, the use of cloud-based platforms for collaboration, and the growing focus on sustainable materials for printing. These trends are transforming how medical devices are developed and produced.
3D Printing Medical Device Software Market Segmentation Details:
| Segmentation | Details |
|---|---|
| Software Type | Design Software, Slicing Software, Scanning Software, Others |
| Application | Prosthetics & Orthopedics, Dental, Medical Implants, Tissue Engineering, Others |
| End-user | Hospitals & Clinics, Medical Device Manufacturers, Research & Academic Institutions, Others |
| Region | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the 3D Printing Medical Device Software Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at