444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview: The 2-valent HPV Vaccine (2vHPV) market is witnessing significant growth due to its pivotal role in cervical cancer prevention. Cervical cancer, primarily caused by high-risk human papillomavirus (HPV) infection, remains a global health concern, driving the demand for effective HPV vaccines. The introduction of the 2vHPV vaccine, targeting HPV types 16 and 18, represents a milestone in the fight against cervical cancer, offering enhanced protection against the most oncogenic HPV strains. With increasing awareness about HPV vaccination and government-led immunization programs, the market for 2vHPV vaccines is poised for expansion in the coming years.
Meaning: The 2-valent HPV Vaccine (2vHPV) is a preventive vaccine designed to protect against infection with HPV types 16 and 18, which are responsible for the majority of cervical cancer cases worldwide. By inducing immune responses against these high-risk HPV strains, the 2vHPV vaccine reduces the risk of HPV-related cervical abnormalities, precancerous lesions, and cervical cancer development.
Executive Summary: The 2vHPV Vaccine market is characterized by increasing vaccine coverage, expanded indications, and strategic partnerships aimed at improving vaccine accessibility and affordability worldwide. Market growth is driven by factors such as rising cervical cancer incidence, growing HPV vaccination awareness, and initiatives to eliminate cervical cancer as a public health threat. As healthcare systems prioritize preventive measures and HPV vaccination becomes an integral component of routine immunization schedules, the market for 2vHPV vaccines is expected to witness sustained growth and innovation.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics: The 2-valent HPV Vaccine market is influenced by factors such as epidemiological trends, vaccination policies, regulatory requirements, and public health priorities, shaping vaccine development, market access, and investment decisions by stakeholders across the healthcare continuum.
Regional Analysis: Regional variations in cervical cancer burden, healthcare infrastructure, regulatory frameworks, and vaccination coverage impact the adoption and utilization of 2vHPV vaccines across different geographic markets. High-demand regions in North America, Europe, and Asia-Pacific exhibit strong market potential for HPV vaccines, driven by factors such as cervical cancer screening programs, HPV awareness campaigns, and government-supported vaccination initiatives.
Competitive Landscape:
Leading Companies in 2-valent HPV Vaccine (2vHPV) Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation: The 2-valent HPV Vaccine market can be segmented based on vaccine type, target population, distribution channel, and geographic region, reflecting variations in vaccine formulation, dosing schedule, and market demand across different age groups, healthcare settings, and patient populations.
Category-wise Insights:
Key Benefits for Public Health:
SWOT Analysis: A SWOT analysis of the 2-valent HPV Vaccine market highlights strengths such as vaccine efficacy, disease prevention, and public health impact, weaknesses related to vaccine access and hesitancy, opportunities for expanded indications and market growth, and threats such as vaccine misinformation and supply chain disruptions.
Market Key Trends:
Covid-19 Impact: The Covid-19 pandemic has underscored the importance of vaccination as a cornerstone of public health preparedness, pandemic response, and disease prevention strategies, emphasizing the need for sustained investment in HPV vaccination programs, healthcare infrastructure, and vaccine confidence-building efforts to address future health challenges and ensure global health security.
Key Industry Developments:
Analyst Suggestions:
Future Outlook: The future outlook for the 2-valent HPV Vaccine market is promising, driven by increasing vaccine uptake, expanding vaccination programs, and advances in vaccine technology, positioning HPV vaccination as a key strategy for cervical cancer prevention, HPV-related disease control, and global health equity. Continued investment in HPV vaccination research, development, and implementation will contribute to achieving ambitious public health goals, reducing cervical cancer burden, and improving health outcomes for generations to come.
Conclusion: In conclusion, the 2-valent HPV Vaccine market represents a dynamic and evolving segment of the global vaccine industry, with significant potential to transform cervical cancer prevention efforts, advance women’s health, and contribute to the achievement of sustainable development goals. By harnessing the power of vaccination, innovation, and collaboration, stakeholders can drive market growth, expand vaccine access, and accelerate progress towards a future free from HPV-related cancers and preventable diseases.
What is 2-valent HPV Vaccine (2vHPV)?
The 2-valent HPV Vaccine (2vHPV) is a vaccine designed to protect against two types of human papillomavirus (HPV) that are responsible for a significant percentage of cervical cancer cases. It works by stimulating the immune system to recognize and fight these specific HPV strains.
What are the key companies in the 2-valent HPV Vaccine (2vHPV) Market?
Key companies in the 2-valent HPV Vaccine (2vHPV) Market include Merck & Co., Inc., and GlaxoSmithKline plc, which are known for their contributions to HPV vaccine development and distribution, among others.
What are the growth factors driving the 2-valent HPV Vaccine (2vHPV) Market?
The growth of the 2-valent HPV Vaccine (2vHPV) Market is driven by increasing awareness of HPV-related diseases, rising vaccination rates, and government initiatives promoting HPV vaccination programs. Additionally, advancements in vaccine technology contribute to market expansion.
What challenges does the 2-valent HPV Vaccine (2vHPV) Market face?
The 2-valent HPV Vaccine (2vHPV) Market faces challenges such as vaccine hesitancy among certain populations, misinformation regarding vaccine safety, and varying healthcare access across different regions. These factors can hinder vaccination efforts and market growth.
What opportunities exist in the 2-valent HPV Vaccine (2vHPV) Market?
Opportunities in the 2-valent HPV Vaccine (2vHPV) Market include expanding vaccination programs in developing countries, increasing partnerships between public health organizations and pharmaceutical companies, and the potential for new formulations that enhance efficacy and coverage.
What trends are shaping the 2-valent HPV Vaccine (2vHPV) Market?
Trends in the 2-valent HPV Vaccine (2vHPV) Market include a growing focus on preventive healthcare, the integration of HPV vaccination into routine immunization schedules, and the development of educational campaigns to improve public understanding of HPV and its vaccines.
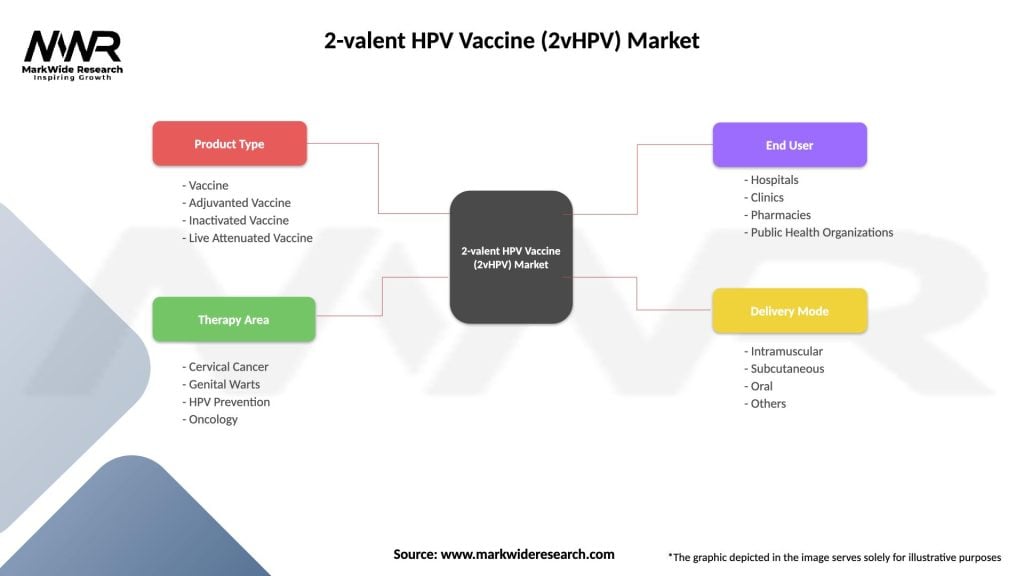
2-valent HPV Vaccine (2vHPV) Market
| Segmentation Details | Description |
|---|---|
| Product Type | Vaccine, Adjuvanted Vaccine, Inactivated Vaccine, Live Attenuated Vaccine |
| Therapy Area | Cervical Cancer, Genital Warts, HPV Prevention, Oncology |
| End User | Hospitals, Clinics, Pharmacies, Public Health Organizations |
| Delivery Mode | Intramuscular, Subcutaneous, Oral, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in 2-valent HPV Vaccine (2vHPV) Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at